Introduction
Irritable bowel syndrome (IBS) is a disorder of gut–brain interaction (DGBI) whose pathophysiology is related to any combination of alterations in motility, visceral sensitivity, epithelial barrier, mucosal immune function, intestinal dysbiosis, or processing at the level of the central nervous system1. Due to its multifactorial nature, diagnosis is based on symptom criteria. In the Global Epidemiological Study of the Rome Foundation, using the most recent Rome IV criteria (see below), it was found that, in Mexico, 40.2% of the general population met criteria for at least one DGBI, and the prevalence of IBS was 4%2. IBS is commonly referred to as the main reason for consultation with a gastroenterologist3,4; however, among subjects reporting symptoms compatible with IBS, only a little more than half sought medical attention, mainly consulting general practitioners, followed by gastroenterologists2. In fact, in a recent study we conducted in Mexico, it was determined that IBS ranked only seventh among reasons for consultation with a specialized gastroenterologist5.
The diagnosis of IBS is based on the application of the Rome criteria, which have evolved according to evidence up to the most recent Rome IV version6, allowing the disorder to be identified from specific symptoms. Nevertheless, due to the overlap of symptoms with those of other intestinal diseases, an adequate evaluation is essential to rule out conditions with similar manifestations, such as inflammatory bowel disease (IBD), celiac disease, or malabsorption disorders1.
In this review, we will analyze the Rome IV criteria for IBS, the Bristol Stool Scale, the differential diagnosis, alarm features, clinical criteria, diagnostic tests and biomarkers, and the multidimensional clinical profile (MDCP). The aim is for this article to serve as a comprehensive guide for the clinician interested in diagnosing IBS, mainly in Mexico, but also for researchers in the field.
Rome IV diagnostic criteria
In the 1980s, DGBIs were referred to as “functional GI disorders,” meaning any condition with gastrointestinal symptomatology when all other possible explanations had been ruled out. As a condition lacking biomarkers, it was viewed as a “diagnosis of exclusion.” A 1988 review article emphasized the intermittency of abdominal pain and the variability of stool consistency in patients7. This was an important precedent for the creation of the Rome Foundation, whose members published their first book (Rome I) in 1994, thereby originating the first diagnostic criteria for the so-called “functional gastrointestinal disorders.” These have been modified three more times in accordance with available evidence: in 2000 (Rome II), 2006 (Rome III), and most recently in 2016 (Rome IV)8. These criteria classify DGBIs into 32 diagnostic categories distributed by target organ: esophageal, gastroduodenal, intestinal, anorectal, biliary tract, and centrally mediated abdominal pain9. IBS belongs to the intestinal disorders and is the most investigated, though not necessarily the most prevalent (currently functional constipation holds that position)10. Of note, the Rome IV criteria are a work in progress, and the new iteration, Rome V, is under development and will be published in May 202611.
Due to the multifactorial nature and the absence of diagnostic biomarkers for IBS—as with other DGBIs—it is necessary that patients first meet the Rome IV diagnostic criteria. However, while some DGBIs are diagnosed exclusively on clinical criteria or symptoms (such as IBS and functional constipation), others require additional diagnostic tests, such as reflux hypersensitivity and functional heartburn, which require pH-impedance monitoring, or pelvic floor dyssynergia/anismus, which requires anorectal manometry12.
As noted above, to date, no reliable biomarkers have been identified for IBS. Although there is intense research to identify them13, no blood, urine, stool, imaging, endoscopy, or biopsy study can replace the use of symptom-based criteria (Rome criteria)14.
The Rome IV diagnostic criteria for IBS are as follows6:
- – Recurrent abdominal pain, on average, at least 1 day per week in the last 3 months; and
- – Associated with at least 2 of the following:
- Defecation (pain improves or worsens).
- A change in stool frequency (more or less frequent).
- A change in stool form (harder or looser than normal).
Symptoms must be present for the last 3 months, with symptom onset at least 6 months before diagnosis6.
It is important to consider the most relevant changes introduced in the Rome IV version vs Rome III. In particular, the most significant change is the elimination of the concept of ”discomfort” from the definition (Rome III required “abdominal pain or discomfort”), with Rome IV now requiring abdominal pain alone, at least once per week, to establish the diagnosis. In Rome III, pain or discomfort had to be present two or more times per month, making the criteria less strict. Moreover, Rome III considered that abdominal pain could only improve with defecation, whereas Rome IV also includes the possibility that pain may worsen with defecation15. Regarding the changes introduced in Rome IV, the modification of the timeframe appears to be the most important factor influencing global IBS prevalence, which decreased by more than 50% from Rome III to Rome IV (10.1% to 4.1%)16.
Secondly, IBS is categorized into 1 of 4 possible subtypes according to the predominant bowel habit: IBS with diarrhea (IBS-D), IBS with constipation (IBS-C), mixed IBS (IBS-M), and unclassified IBS (IBS-U). For classification, it is necessary to consider the type of abnormal bowel movements that predominate in the patient, using a 25% threshold during days with abnormal stools (Table 1). This means that if a patient reports altered bowel movements (liquid, very hard, or both) on 5 of 7 days per week, the 5 days are considered as the 100% basis; conversely, if abnormal evacuations occur all 7 days, then the 7 days are taken as the 100% basis6.
Table 1. Subtypes of irritable bowel syndrome according to Rome IV
| Subtype | Criteria | Patients |
|---|---|---|
| IBS-D | At least 25% of stools are Bristol types 6 or 7, and < 25% are Bristol types 1 and 2 | Report that abnormal bowel movements are usually diarrhea |
| IBS-C | At least 25% of stools are Bristol types 1 or 2, and < 25% are Bristol types 6 and 7 | Report that abnormal bowel movements are usually constipation |
| IBS-M | At least 25% of stools are Bristol types 1 and 2, and at least 25% are Bristol types 6 or 7 | Report that abnormal bowel movements are usually both constipation and diarrhea |
| IBS-U | Meets diagnostic criteria for IBS, but no stool form predominates beyond 25% | Report that abnormal bowel movements are rare |
|
D: diarrhea; C: constipation; M: mixed; U: unclassified; IBS: irritable bowel syndrome. |
||
Bristol stool form scale
The IBS subtype is based primarily on the Bristol Stool Form Scale, which distinguishes seven stool types according to intestinal transit (Fig.1).
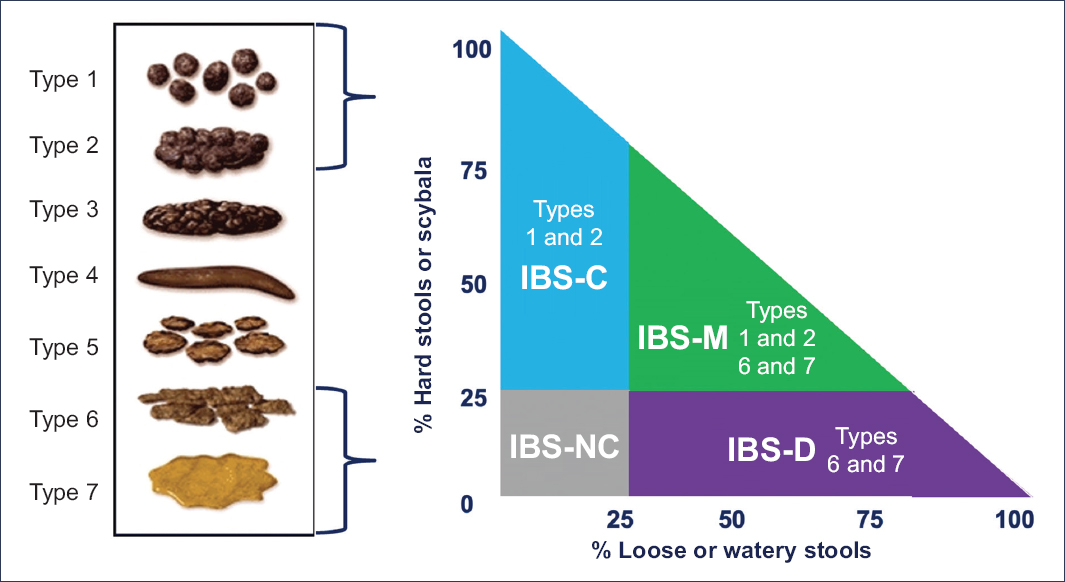
Figure 1. Bristol stool form scale.
Differential diagnosis
A large number of organic disorders may meet the same criteria as IBS; however, there are associations more common in IBS than in organic disease, which support the diagnosis of IBS (Table 2)17. Examples: an unpredictable bowel habit (≥ 3 stool forms per week) is more common in IBS-D, while a greater number of consecutive days without evacuation is more strongly associated with IBS-C. Urgency and mucus in stools are more common in IBS-D, while excessive straining and incomplete evacuation are more common in IBS-C18. Extraintestinal digestive symptoms may include dyspepsia, early satiety, nausea, epigastric pain, and postprandial fullness18,19. In a Mexican study we conducted in patients with IBS (Rome III criteria), those with IBS-M reported higher frequency and intensity of halitosis, vomiting, and belching, while IBS-C patients had more straining, and IBS-D patients had more urgency, fecal incontinence, and mucus in stools20. These symptoms occur in up to one-third of patients and are correlated with increased work disability and need for medical care21.
Table 2. Differential diagnoses of irritable bowel syndrome in the routine clinical practice
| Chronic diarrhea | Gastrointestinal | Celiac disease |
| Small intestinal bacterial overgrowth | ||
| Chronic infection | ||
| Colonic neoplasia | ||
| Inflammatory bowel disease | ||
| Ulcerative colitis | ||
| Crohn’s disease | ||
| Other | Hyperthyroidism | |
| Hypoparathyroidism | ||
| Diabetes | ||
| Drugs (proton pump inhibitors, prokinetics, metformin, colchicine) | ||
| Chronic constipation | Gastrointestinal | Functional constipation |
| Opioid-induced constipation | ||
| Functional defecation disorders Defecatory dyssynergia | ||
| Inadequate defecatory propulsion | ||
| Slow-transit constipation | ||
| Defecatory disorders | ||
| Other | Drugs (calcium channel blockers, nonsteroidal anti-inflammatory drugs) | |
| Parkinson’s disease | ||
| Diabetes mellitus | ||
| Connective tissue diseases | ||
| Ehlers-Danlos syndrome | ||
| Mood disorders |
Extraintestinal symptoms include fibromyalgia, chronic fatigue syndrome, chronic pelvic pain, temporomandibular joint disorder, headache, neck and back pain, myalgias, fatigue, dizziness, migraine, palpitations, chest pain, hot flashes, sleep disorders, decreased libido, dyspareunia, urinary urgency and frequency, nocturia, anxiety, depression, dyspnea, asthma, cough, pruritus, and halitosis22,23.
Alarm features
As mentioned above, the diagnosis of IBS is based on information obtained from the medical history, and patients must meet the criteria defined by Rome IV. Of note, up to 24% of patients with organic diseases may fulfill Rome criteria, including inflammatory bowel disease, celiac disease, lactose intolerance, and microscopic colitis, among others24. Therefore, a complete physical examination must be performed to rule out alarm features and to reassure the patient that no other disease is present. Alarm features that should be systematically sought include unintentional weight loss (>10% in 3 months), blood in the stool not caused by hemorrhoids or anal fissures, predominantly nocturnal diarrhea, fever, and family history of inflammatory bowel disease or celiac disease6. For example, in IBS-D, the absence of alarm features reduces the likelihood ratio of an organic disease25, although alarm features have low sensitivity and specificity for the diagnosis of colorectal cancer26. Thus, the selection of diagnostic tests should be individually guided by the specific clinical context6,27.
Accordingly, once the Rome IV diagnostic criteria for IBS are confirmed, and given that ruling out an organic cause for symptoms is fundamental, it is necessary to determine precisely which complementary studies are required to confirm the functional nature of the disorder (see sections on biomarkers and the MDCP).
Clinical criteria
The Rome criteria have high sensitivity for diagnosing IBS based on symptoms. These criteria are of particular value for epidemiological research, pathophysiological studies, and clinical trials1,8. While they serve to guide diagnosis in clinical practice, they can be difficult to apply in real-world settings, thus posing a challenge for physicians and gastroenterologists. This difficulty arises because many patients do not meet the required symptom timeframe, a situation referred to as a ”subthreshold diagnosis.” Nevertheless, this group of patients often receives the same treatment as those who fully meet the criteria28. Of note, in general, patients suspected of having IBS seek medical care when their symptoms are bothersome enough to affect their daily lives29. In this context, and in view of the limitations of the Rome criteria for clinical application, the Board of Directors of the Rome Foundation developed, by consensus, a modification of the Rome IV diagnostic criteria for use in clinical practice, known as the “clinical criteria”8. Four factors were proposed for these clinical criteria:
- – Nature of symptoms: Symptoms must fulfill the qualitative characteristics of the Rome IV criteria, which have been supported and validated by epidemiological, factorial analysis, and clinical cohort studies, among others.
- – Distress/interference with daily life: It has been recommended to consider as a clinical criterion the patient’s own report that symptoms are sufficiently bothersome to interfere with activities of daily life.
- – Frequency of symptoms: Symptom frequency should not be considered a mandatory criterion for IBS diagnosis, since patients usually seek medical consultation because symptoms impact their daily lives, even if frequency is below the Rome IV threshold.
- – Duration of symptoms (timeframe): While Rome IV requires symptoms during the last 3 months, with onset at least 6 months before diagnosis, it is acceptable to consider symptoms present within the past 8 weeks, provided that other diagnoses have been excluded. Two exceptions to this duration requirement exist: a) when the physician needs to make an early diagnosis and is confident that other diseases have been excluded; and b) in disorders where symptoms occur infrequently and intermittently (eg, cyclic vomiting syndrome, abdominal migraine, biliary pain, or proctalgia fugax).
Although these guidelines are recommended to improve the implementation of the Rome criteria in clinical practice, physicians must still evaluate symptom patterns, risk factors, and other patient characteristics to determine whether additional testing is necessary. If all elements are consistent with IBS, the diagnosis can be established with confidence, even with lower frequency and shorter duration of symptoms.
Minimal diagnostic tests and biomarkers
Currently, the only recommended clinical use of biomarkers in IBS consists of serological tests for tissue transglutaminase immunoglobulin A (IgA), total IgA, blood C-reactive protein (CRP), and fecal calprotectin30. These markers do not confirm the diagnosis of IBS (inclusion biomarkers) but are instead used to exclude celiac disease and inflammatory bowel disease in patients with suspected IBS-D (exclusion biomarkers) and atypical clinical features or absence of alarm signs. For this reason, in the absence of a specific inclusion biomarker, the diagnosis of IBS remains symptom-based6. Nonetheless, although the Rome criteria have proven useful in clinical trials, they may show certain limitations in daily medical practice due to the clinical heterogeneity of patients, as well as the overlap of symptoms with those of other conditions31.
If IBS diagnosis were to be based strictly on a “diagnosis of exclusion,” patient work-up could be so extensive that it would take months or even years to establish the correct diagnosis32, since physicians would need to exclude IBD, celiac disease, food intolerances (fructose, lactose), and even GI tumors33. Current recommendations state that early diagnosis should be made based on symptoms, with limited use of extensive, costly, or invasive testing34. Nonetheless, many patients continue to undergo numerous diagnostic studies, which delay both diagnosis and appropriate treatment. For example, in a study conducted among gastroenterologists who are members of the Latin American Society of Neurogastroenterology—experts in IBS diagnosis—98% reported using Rome IV criteria to diagnose IBS. All ordered laboratory tests in the presence of alarm signs, and 90% requested colonoscopy in patients over 50 years, as established in various international guidelines. Despite this, 73% ordered abdominal–pelvic CT scans, demonstrating overuse of unnecessary studies for diagnosis35. This highlights the crucial importance of investigating specific biomarkers through accessible, minimally invasive procedures that increase diagnostic precision and support improved treatment strategies for IBS.
Of note, a biomarker is defined as an objective characteristic that serves as an indicator of normal or pathological biological processes, or of responses to an exposure or intervention36. Biomarkers may have different natures—from molecular to histological, radiological, or imaging features, or physiological characteristics—and may be applied in different contexts: screening, diagnosis, monitoring, pharmacodynamics, therapeutic response, prediction, or prognosis36. In other words, the ideal biomarker should measure a biological component, structure, or function that influences or can predict the course of a disorder or disease37. Similarly, it should have high sensitivity and specificity, reproducibility, cost-effectiveness, low interobserver variability, and accessibility for both the healthcare system and the patient38. Searching for biomarkers that meet these features or purposes is of vital importance for IBS diagnosis and management.
To date, no specific biomarker exists for IBS diagnosis, but some have been studied as supportive tools. In 2009, Lembo et al39 published a study developing and validating a diagnostic test based on serum biomarkers to differentiate IBS patients from those with other GI diseases and from healthy subjects39. The study included 10 biomarkers: interleukin-1β (IL-1β), growth-related oncogene-α (GRO-α), brain-derived neurotrophic factor (BDNF), anti-Saccharomyces cerevisiae antibody (ASCA IgA), anti-flagellin CBir1 antibody (anti-CBir1), anti–tissue transglutaminase antibody (anti-tTG), TNF-like weak inducer of apoptosis (TWEAK), antineutrophil cytoplasmic antibody (ANCA), tissue inhibitor of metalloproteinases-1 (TIMP-1), and neutrophil gelatinase–associated lipocalin (NGAL). The test showed 50% sensitivity, 88% specificity, a positive predictive value of 81%, and a negative predictive value of 64%. Although overall sensitivity was low, the high specificity suggests that a positive result could improve diagnostic accuracy for IBS39. Finally, this serum biomarker panel may be useful as an adjunct early in clinical evaluation, particularly in atypical presentations and to avoid unnecessary invasive tests, especially in distinguishing IBS from nonfunctional GI disorders. However, this panel does not replace clinical assessment and should not be used in isolation39.
Then, Jones et al40 proposed a panel of 34 biomarkers combined with psychological variables (anxiety, depression, somatization, stress) to differentiate IBS patients from healthy controls and among IBS subtypes. This test added a broader, modern approach incorporating the 10 biomarkers from Lembo’s study39 plus 24 new ones, including 14 gene-expression markers in peripheral blood and 10 new serological markers. The 34-biomarker panel demonstrated 81% sensitivity and 64% specificity, and when combined with standardized psychological assessments, performance improved up to ≥ 85% for both sensitivity and specificity. Moreover, this panel was able to discriminate effectively between IBS-C and IBS-D40. Among the most useful markers identified in this study were histamine, related to mast cell activation; anti-tTG, a marker of antibody expression; and NGAL, involved in mucosal regeneration and molecular transport. Additional markers evaluated included IL-6, an important inflammatory mediator; vasoactive intestinal peptide receptor 1 (VIPR1), associated with inflammation and motility; and TWEAK, involved in inflammation, motility, and tissue repair. Furthermore, gene-expression markers such as RNF26 (ring finger protein 26), ZNF326 (zinc finger protein 326), and MICALL-1 (MICAL-1–like gene) were analyzed, all of which are associated with tight junctions and epithelial barrier function40. Thus, the panel proposed by Jones et al40 evaluates biomarkers related to multiple pathophysiological mechanisms of IBS, including low-grade inflammation, epithelial barrier dysfunction, neuroimmune alterations, mast cell activation, and peripheral gene regulation. In addition, it integrates genes and proteins that, when combined with psychological variables, reinforce the biopsychosocial model of IBS. Therefore, this study proposes a model that differentiates IBS from healthy individuals as well as from other organic gastrointestinal diseases, with adequate accuracy40.
On the other hand, certain studies have demonstrated elevated levels of proinflammatory cytokines, such as tumor necrosis factor alpha (TNF-α), as well as decreased levels of the anti-inflammatory cytokine IL-10, in the blood of patients with IBS compared with healthy subjects41. Similarly, it has been reported that patients with immune alterations also experience faster intestinal transit compared with those without immune activation42. Moreover, a positive correlation exists between TNF-α levels and decreased stool consistency, while elevated IL-6 levels are associated with increased bowel frequency42.
In post-infection IBS (PI-IBS) models, such as rats infected with Campylobacter jejuni, a phenotype similar to IBS-D has been observed, along with changes to the small-intestinal microbiota43. A key finding in this model was the role of the bacterial cytolethal distending toxin subunit B (CdtB)44. Exposure to CdtB induced the production of specific antibodies, which through cross-reactivity with vinculin—a cellular adhesion protein in the intestine—was linked to altered motility and subsequent small-intestinal bacterial overgrowth43–45. This led to the development of a serological test for anti-CdtB and antivinculin antibodies45.
As noted, biomarkers may be useful not only to distinguish IBS from healthy subjects, but also to differentiate subgroups within IBS. One example is patients who develop IBS following GI infection (PI-IBS). Approximately 10% of individuals who experience acute gastroenteritis subsequently develop persistent symptoms consistent with IBS-D, which characterizes PI-IBS46–48. Based on this preclinical evidence, in 2015 Pimentel et al45 conducted a clinical study to evaluate whether anti-CdtB and antivinculin antibodies could be used as diagnostic biomarkers for IBS-D and to differentiate it from other causes of chronic diarrhea, such as celiac disease, inflammatory bowel disease, and from healthy controls. The study found significantly elevated levels of both antibodies in patients with IBS-D. In particular, anti-CdtB antibodies showed good diagnostic performance, with an area under the curve of 0.81, specificity of 91.6%, and a positive predictive value of 81%45. In line with these findings, a study in a Mexican population by Schmulson et al49 assessed the clinical utility of anti-CdtB and antivinculin antibodies as diagnostic tools in patients with IBS-D and IBS-M. Positivity for at least one of these antibodies was found in 58.8% of IBS-D patients and 33.3% of those with IBS-M. Moreover, patients with a past medical history of PI-IBS showed a higher positivity rate (71.4%) vs non-post-infectious cases (41.7%); although not statistically significant, this finding supports the hypothesis of an immune-mediated mechanism triggered by prior infections49. Notably, these biomarkers were not positive in patients with other functional or organic causes of diarrhea, underscoring their diagnostic specificity. These findings support the potential use of anti-CdtB and antivinculin antibodies as complementary tools for the positive diagnosis of IBS-D and to distinguish it from other chronic diarrhea conditions49. These are perhaps the most widely used inclusion biomarkers in clinical practice. In the authors’ experience, these biomarkers should not replace the Rome diagnostic criteria, but they are useful when patients require objective test results to accept their diagnosis.
Fecal biomarkers are also a noninvasive diagnostic tool in the evaluation of GI disorders. One of their most relevant advantages is their ability to detect inflammatory activity in the intestinal mucosa, playing an important role in distinguishing IBS from organic diseases such as inflammatory bowel disease (IBD)37. An example is fecal calprotectin, the most widely studied parameter for evaluating intestinal inflammation42, with a sensitivity of 93% and a specificity of 94% for differentiating IBS from IBD when using 50 μg/g of stool as the cutoff point50,51. Therefore, a negative fecal calprotectin result would effectively rule out IBD in a patient with suspected IBS, reducing the need for invasive or costly tests such as colonoscopy.
Another important biomarker worth evaluating in patients with IBS-D is fecal bile acids for the assessment of bile acid malabsorption52. Under physiological conditions, bile acids are reabsorbed in the ileum and return to the liver via enterohepatic circulation52. When this process is disrupted, bile acids can stimulate colonic motility, secretion, and intestinal permeability, leading to diarrhea52. Some studies suggest that bile acid malabsorption accounts for up to 30% of IBS-D cases53,54. The gold standard diagnostic test is the 75selenium-homotaurocholic acid (75SeHCAT) retention test; however, this is not available in Mexico55. Alternatively, 48-hour fecal bile acid quantification is often used, though the cost may reach up to USD 825, and samples must be sent abroad for analysis55. Consequently, serum levels of 7αC4 (a metabolite derived from 7α-hydroxylase, the rate-limiting enzyme in bile acid synthesis) have shown favorable results for screening bile acid malabsorption, representing up to a 50% cost reduction for patients in Mexico, according to local experience55. Although bile acid malabsorption is not the sole cause of IBS-D, investigating this condition represents an important diagnostic and therapeutic strategy, as patients may benefit from bile acid sequestrants56.
The study of short-chain fatty acids (SCFAs) has emerged as a promising approach in the evaluation of intestinal microbiota alterations in IBS.57 These include acetate, propionate, and butyrate, microbial fermentation products in the gastrointestinal tract, whose levels may influence intestinal inflammation57. Farup et al58 reported altered butyrate and propionate levels in IBS patients vs controls, with a sensitivity of 92% and specificity of 72%, using a cutoff > 0.015 mmol/L; thus, an increased propionate-to-butyrate difference may reflect dysbiosis or altered bacterial fermentation in IBS patients. Other studies have assessed the clinical utility of combining fecal and blood biomarkers for IBS diagnosis, though this lies beyond the scope of this review59.
Research into novel biomarkers for IBS is ongoing. Fecal metabolomic analysis has emerged as a promising tool for identifying IBS biomarkers, with notable candidates including chromogranin A and secretogranin III60,61. Another promising line of research is volatilomics, focusing on volatile organic compounds, low-molecular weight, highly volatile metabolites62. Microbiome studies have also gained relevance, identifying altered patterns such as increased Firmicutes and decreased Bacteroides in IBS patients63–65. Similarly, microRNAs (miRNAs) have emerged as potential biomarkers due to their regulatory role in gene expression; for instance, miRNA-24 reduces expression of the serotonin transporter, a mechanism associated with increased IBS symptom severity66.
Since no single biomarker for IBS has yet been identified, symptom-based diagnosis using the Rome IV criteria remains fundamental. Nonetheless, the integration of metabolomic, microbial, immunologic, and genetic data represents a promising pathway toward personalized medicine. Validation of these biomarkers across diverse populations and their incorporation into clinical practice will enable more accurate diagnoses, better follow-up, and more effective therapeutic strategies for patients with IBS.
Diagnostic algorithm and multidimensional clinical profile
Since there is no biomarker or specific test to confirm or rule out the diagnosis of IBS, the Rome IV guidelines state that the diagnosis of IBS requires a meticulous approach, limited diagnostic testing, and careful follow-up29. For this purpose, the Rome Foundation developed diagnostic algorithms for GI symptoms67, providing a practical, efficient, and cost-effective method to diagnose common GI disorders. These algorithms begin with GI symptoms; for example, in IBS, they start with recurrent abdominal pain associated with altered bowel habits, followed by the necessary diagnostic tests, and ending with the diagnosis of IBS and its subtypes. This information is the first step in the clinician’s decision-making process for establishing the diagnosis of a disorder of DGBI. Figure 2 illustrates the diagnostic algorithm for IBS.
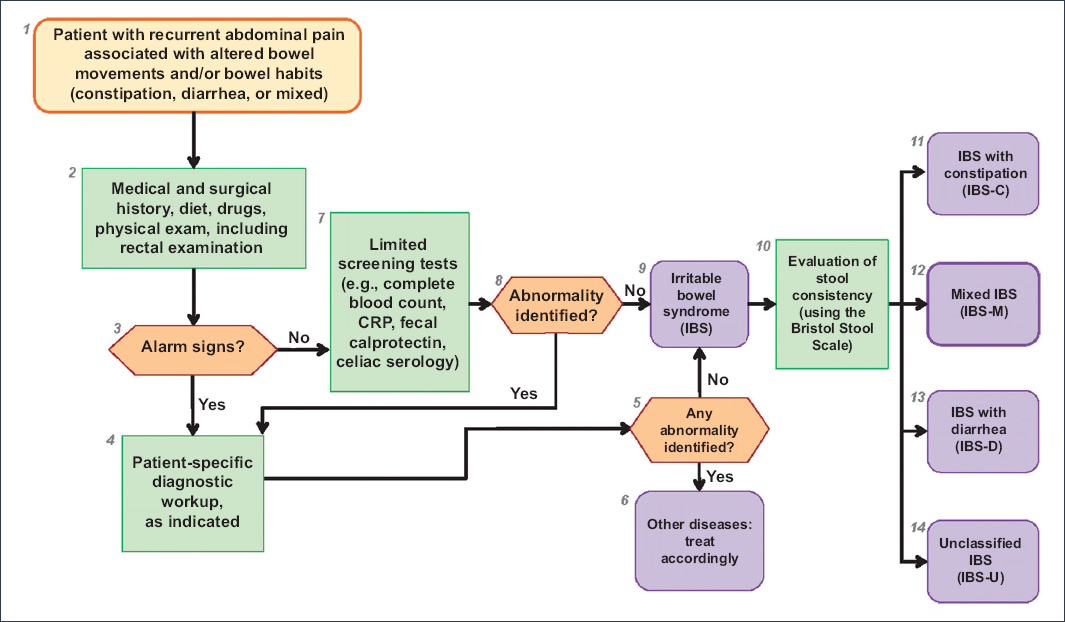
Figure 2. Rome IV diagnostic algorithm for recurrent abdominal pain with altered bowel habits. CRP: C-reactive protein.
The second component is the therapeutic management of IBS. Although the Rome IV criteria provide a solid foundation for reaching a consistent diagnosis of IBS, by themselves they do not encompass all the dimensions of the patient’s clinical status. For this reason, the Rome Foundation developed the MDCP, which captures the full range of signs in patients with DGBIs, thereby allowing individualized treatment for each case68. The MDCP includes 5 categories with emphasis on IBS:
- – Categorical diagnosis: Rome IV criteria for IBS.
- – Clinical modifiers: IBS subtype (IBS-C, IBS-D, IBS-M, IBS-U, and PI-IBS); FODMAP (fermentable oligo-, di-, monosaccharides, and polyols) sensitivity; gluten sensitivity; and presence of subjective or objective/visible abdominal distension.
- – Self-perceived severity or impact on daily life: mild, moderate, or severe, according to the question: ”How much does the disorder affect your daily life?”
- – Psychosocial modifiers: may be categorical (according to the Diagnostic and Statistical Manual of Mental Disorders, 5th edition), dimensional (Hospital Anxiety and Depression Scale [HADS], psychological alarm signs such as anxiety or depression), or patient-reported (history of physical or sexual abuse).
- – Physiological modifiers and clinically relevant biomarkers: physiological or biochemical parameters that improve diagnostic understanding or influence management (eg, anorectal manometry; colonic transit studies; visceral sensitivity via barostat; evidence of inflammation from biochemistry, histology, fecal calprotectin, cytokines, mRNA, or celiac serology; autoimmunity markers such as anti-CdtB and antivinculin; and other tests such as bile acid malabsorption, intestinal permeability, fecal tryptase, and intestinal microbiota). It should be noted that in IBS, biomarker application remains very limited, as previously mentioned.
Let us consider an example of the application of the MDCP in a case report of IBS. This is a 32-year-old woman, single, employed in a law firm, who presented with a 2-year history of diarrhea along with abdominal pain, bloating, and occasional flatulence. The symptoms had been intermittent, but she reported that over the last year they had become more frequent and increasingly interfered with her quality of life. She reported abdominal pain at least 3 days per week, which was exacerbated by work-related stress, especially during tax season deadlines. The pain was relieved by bowel movements and was occasionally accompanied by subjective abdominal distension. She further noted that at least 50% of her bowel movements were abnormal and corresponded to loose stools, sometimes associated with urgency. In recent months, she also reported difficulty concentrating, a sensation of “brain fog” or mental slowness, which she described as frustrating and at times interfering with her work performance. These episodes tended to coincide with days of greater digestive discomfort. The patient stated that these symptoms had moderately affected her daily activities. On directed questioning, she reported that for the past year she had experienced increased stress, as she had begun preparations for her wedding. Her HADS score was 11 for anxiety and 5 for depression. Previous laboratory tests, including thyroid panel, celiac serology, fecal calprotectin, and Giardia antigens all turned out negative. Upper endoscopy and colonoscopy with biopsies were normal, showing no evidence of intestinal malabsorption or microscopic colitis. On physical examination, vital signs were within normal ranges, body mass index was 22.1 kg/m2, and there was abdominal tenderness without peritoneal irritation or palpable masses. There were no alarm features or family history of inflammatory bowel disease, celiac disease, or colon cancer. A 7αC4 test was ordered and returned positive. Clinical findings were consistent with IBS-D according to the Rome IV criteria in a young patient, with no alarm features, no relevant family history, and negative testing for organic causes. The clinical course, along with exclusion of other etiologies such as celiac disease, microscopic colitis, parasitic infections, and inflammatory bowel disease, supported this initial suspicion. Similarly, the positive 7αC4 result suggested the presence of bile acid malabsorption, a functional disorder underlying IBS-D in up to 30% of these patients53. Overall, the clinical presentation, the impact on quality of life, and the positive 7αC4 test point toward a diagnosis of IBS-D due to bile acid malabsorption. The explanation of the MDCP categories for this case is presented in Table 3. The MDCP allows us to categorize this patient as IBS-D with subjective bloating and urgency, moderate severity, anxiety and emotional stress, and bile acid malabsorption evidenced by 7αC4. Although this review is not intended to address treatment, it should be noted that in this case empirical treatment with bile acid sequestrants, such as cholestyramine or colesevelam, would be recommended, with follow-up to assess clinical response. Monitoring of psychological symptoms and emotional impact is also advised, given the frequent interaction between gastrointestinal symptoms and the psychosocial status of these patients.
Table 3. Example of a clinical case and application of the multidimensional clinical profile
| Category | Explanation of the clinical case |
|---|---|
| Category A: categorical diagnosis | The patient meets the Rome IV criteria for IBS, as she has abdominal pain at least once a week related to defecation and changes in stool appearance, with symptoms lasting > 6 months |
| Category B: clinical modifiers | IBS-D, since more than 25% of bowel movements are liquid and less than 25% are hard, according to the Bristol scale. In addition, the patient reports additional symptoms, such as subjective abdominal distension, bloating, flatulence, and occasional urgency to defecate, which, although not part of the diagnostic criteria, are relevant to the clinical and therapeutic approach |
| Category C: personal impact | The patient responded “moderately” to the question: “Overall, to what extent do your symptoms interfere with your activities (work, school, social activities, self-care, concentration, and performance)?”. This level of impact should be considered when deciding on the intensity of treatment and the need for a comprehensive approach |
| Category D: psychosocial modifiers | Clinical anxiety and emotional stress associated with multiple stressors. The stress of planning her wedding appears to be a triggering and perpetuating factor for her symptoms in the context of the gut-brain axis. These factors are relevant for the design of therapeutic strategies that include psychoeducational components or psychotherapeutic interventions |
| Category E: physiological modifiers and biomarkers | 7αC4 positive: indicates bile acid malabsorption. This finding allows for more accurate stratification according to pathophysiology and a specific therapeutic opportunity through the use of bile acid sequestrants |
|
D: diarrhea; IBS: irritable bowel syndrome. |
|
Conclusions
The diagnosis of IBS is fundamentally based on the Rome IV criteria, which identify patients through cardinal symptoms such as recurrent abdominal pain associated with altered bowel habits and stool consistency. These criteria were developed to standardize patient selection for research, whether epidemiologic studies or clinical trials. In clinical practice, they should be used as a diagnostic guide, although clinical criteria based on Rome IV allow shortening of the diagnostic timeline when physicians are confident based on prior evaluations. Additionally, the Rome Foundation has developed diagnostic algorithms to guide the evaluation of patients with different DGBIs. Finally, by encompassing all relevant dimensions of DGBIs, the MDCP allows a comprehensive characterization of patients, facilitating the choice of personalized therapeutic strategies and optimizing long-term outcomes. In this context, the development of biomarkers may contribute in the future to better patient characterization. Although biomarkers have not yet replaced clinical criteria (Rome IV), their validation and progressive incorporation into medical practice may transform the diagnostic and therapeutic approach to IBS.
Funding
This work was partially funded by the Research Division of the School of Medicine, Universidad Autónoma de Mexico. G. Mendoza-Domínguez declared having received a postgraduate fellowship from SECIHTI (CVU: 2094907). A.S. Morales-Guzmán declared having received a postgraduate fellowship from SECIHTI (CVU: 1141922).
Conflicts of interest
C.L. Cruz-Rico, G. Mendoza-Domínguez, S.A. Zaragoza-Galicia, and A.S. Morales-Guzmán declare no conflicts of interest. M.J. Schmulson: Advisory Board for Daewoong Korea, Gemelli Biotech Inc, Moksha 8 Mexico, Pro.Med.CS Prague; speaker for Alfa Sigma Mexico, Armstrong Mexico, Carnot, Daewoong Korea, Ferrer Mexico/Central America, Medix Mexico, Megalabs, Moksha 8 Mexico, Ecuador, Tecnofarma Colombia/Bolivia; educational materials for Moksha 8 Mexico.
Ethical considerations
Protection of humans and animals. The authors declare that no experiments on humans or animals were conducted for this review.
Confidentiality, informed consent, and ethical approval. This review does not involve personal patient data and does not require ethical approval. The SAGER guidelines do not apply.
Declaration on the use of artificial intelligence. The authors declare that artificial intelligence was used in the preparation of this manuscript. ChatGPT was employed for drafting the abstract in order to meet the established word limit, as well as for assistance in writing and style editing in the section on biomarkers and diagnostic tests. All content was carefully reviewed by the authors. Artificial intelligence was not used for data collection, analysis, or figure generation.
