Introduction
Irritable bowel syndrome (IBS) is a disorder of gut–brain interaction (DGBI), symptom-based, defined by the Rome IV criteria, and characterized primarily by recurrent abdominal pain and alterations in stool frequency or consistency1. Its global prevalence varies widely, from as low as 1% in France to nearly 35% in Mexico. It is estimated that between 5% and 10% of the Western population is affected2,3.
Beyond its high prevalence, IBS exerts a considerable impact on quality of life. Although historically considered a functional disorder without structural basis, it is now recognized that multiple pathophysiologic mechanisms underlie its clinical expression, explaining its frequent coexistence with other digestive and extra-digestive disorders.
In this context, the concept of overlap refers to the simultaneous presence of symptoms or diagnostic criteria for 2 or more DGBI in the same patient. This phenomenon may include digestive conditions such as functional dyspepsia (FD) or functional constipation (FC), as well as extra-digestive comorbidities such as fibromyalgia (FM), chronic fatigue syndrome (CFS), headache, sleep disorders, and urogenital conditions.
This article reviews the overlap of IBS with other DGBI and associated comorbidities, emphasizing its clinical relevance, shared pathophysiologic foundations, and management strategies.
Definition of overlap in gastroenterology
In gastroenterology, the term “overlap” refers to the coexistence of symptoms or diagnostic criteria corresponding to 2 or more DGBI in the same patient. This phenomenon is not uncommon and represents both a diagnostic and therapeutic challenge, associated with increased health care costs and a significant deterioration in quality of life.
Overlap also poses challenges for research and clinical practice, as patients often present with symptoms that cannot be explained by a single diagnosis. Therefore, an individualized, multidimensional, patient-centered approach is required, addressing both digestive and extra-digestive aspects4.
Clinical importance of overlap in irritable bowel syndrome
Overlap among different DGBI and other comorbidities is a frequent finding in patients with IBS and has substantial clinical implications. A significant proportion of these patients meet criteria for more than one DGBI, present with extra-digestive comorbidities, or experience symptoms in different anatomic regions (Fig. 1).
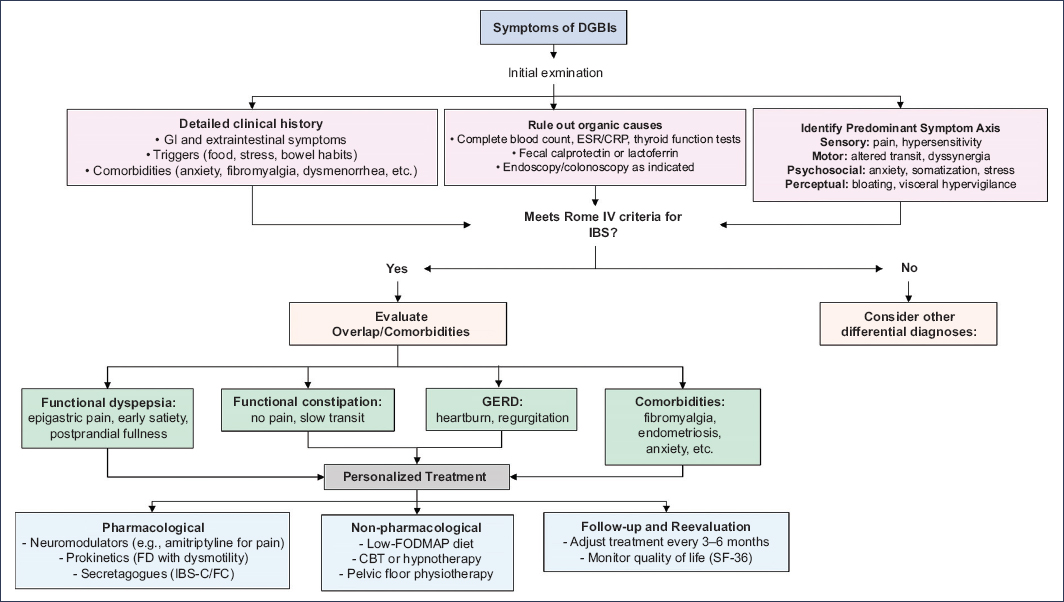
Figure 1. Clinical management algorithm for patients with symptoms compatible with disorders of gut–brain interaction (DGBIs) and possible overlap. FD: functional dyspepsia; FC: functional constipation; GERD: gastroesophageal reflux disease; FM: fibromyalgia; FODMAP: fermentable oligosaccharides, disaccharides, monosaccharides, and polyols; CRP: C-reactive protein; IBS: irritable bowel syndrome; IBS-C: IBS with constipation; CBT: cognitive behavioral therapy; ESR: erythrocyte sedimentation rate.
The Rome Global Epidemiology Study5 reported that more than 30% of the general population meeting criteria for a DGBI under Rome IV had symptoms in 2 or more anatomic regions. In tertiary care settings, this figure may exceed 47%, while in the general population it approaches 26.5%. This suggests that patients managed in specialized units tend to present more complex and refractory conditions.
Overlap of DGBI is associated with greater severity of GI symptoms, poorer quality of life, higher psychological burden (anxiety, depression, somatization), and lower response to first-line therapies. For example, patients with IBS and FD often experience greater symptom intensity and higher emotional burden compared with those presenting a single DGBI. This clinical complexity reinforces the need to identify overlap from the initial evaluation and to adapt the diagnostic–therapeutic approach in an integrated manner, considering all symptomatic axes involved.
In Mexico, the SIGAME Study (Síntomas Gastrointestinales en México)6 represents the first population-based epidemiologic assessment focused on functional GI disorders, including a specific analysis of overlap. This study, conducted in more than 3,900 adults from diverse geographic regions and socioeconomic strata, showed a prevalence of IBS of 7.6%, while FC and uninvestigated dyspepsia reached prevalences of 22.3% and 12%, respectively. These data confirm that DGBI are highly prevalent in the Mexican population, and that a substantial proportion of patients experience symptoms consistent with more than one functional diagnosis.
In particular, the chapter dedicated to overlap in the SIGAME Study documented that the coexistence of IBS with other functional GI disorders is common. Overlap with symptoms of gastroesophageal reflux disease (GERD) occurred in 67 subjects with IBS (22.3%; 95% CI, 17.9-29.2), with dyspeptic symptoms in 54 subjects (17.9%; 95% CI, 14-22.6), and excessive belching in 37 subjects (12.2%; 95% CI, 9-16). These data reinforce the need for a comprehensive, multidimensional evaluation from the first clinical encounter.
Although the findings of SIGAME are consistent with those reported in international studies, they add the value of being based on a representative sample of the Mexican general population, evaluated with validated tools (Rome III, PAGI-SYM, PAGI-QoL). Furthermore, higher frequencies of overlap were identified among women, young adults, and individuals of working age, highlighting the need for diagnostic and therapeutic strategies sensitive to the sociodemographic context.
Incorporating these data into clinical practice strengthens a patient-centered approach, avoids unnecessary treatments and investigations, and favors interventions directed at the predominant symptomatic axis. This approach is essential for improving quality of life, reducing emotional burden, and optimizing the use of resources in health systems with structural limitations such as ours.
Pathophysiologic considerations
DGBIs share pathophysiologic mechanisms that may coexist and potentiate one another, facilitating chronicity and symptom severity. The most relevant include visceral hypersensitivity, motor dysfunction, low-grade immune activation, intestinal dysbiosis, increased epithelial permeability, and alterations in central pain modulation1,7 (Table 1).
Table 1. Shared pathophysiological mechanisms in disorders of gut–brain interaction and their clinical expression
| Pathophysiological mechanism | Symptoms or clinical signs | Associated digestive disorders | Associated extra-digestive comorbidity |
|---|---|---|---|
| Visceral hypersensitivity | Abdominal pain, bloating, urgency, sensation of incomplete evacuation | IBS, FD, FC, FABD | FM, chronic pelvic pain |
| Motility alterations | Postprandial fullness and satiety, nausea, constipation, diarrhea | IBS, FD, FC, gastroparesis, GERD | Migraine, IC |
| Intestinal dysbiosis | Flatulence, bloating, abdominal discomfort, systemic symptoms | IBS, FD, SIBO, PI-IBS | CFS, anxiety, mood disorders |
| Low-grade inflammation | Persistent pain, hyperalgesia, nonspecific chronic symptoms | IBS, FD, PI-IBS | CFS, FM |
| Increased intestinal permeability | Abdominal discomfort, systemic inflammation, multi-organ symptoms | IBS, dysbiosis | Endometriosis |
| Gut-brain axis dysfunction | Anxiety, depression, insomnia, pain amplification, dysautonomia | All DGBIs | Mood disorders, sleep disorders |
| Central sensitization | Widespread pain, exaggerated response to mild stimuli | IBS, FD | FM, chronic headache |
| Altered neurological modulation | Circadian rhythm disturbances, appetite changes, hyperalgesia | IBS, FD, FC | Sleep disorders, anxiety, depression |
|
IC; interstitial cystitis; FABD, functional abdominal bloating/distension; FD; functional dyspepsia; FC, functional constipation; GERD; gastroesophageal reflux disease; CFS; chronic fatigue syndrome; FM; fibromyalgia; SIBO, small intestinal bacterial overgrowth; IBS, irritable bowel syndrome; PI-IBS; post-infectious IBS; DGBIs, disorders of gut–brain interaction. |
|||
Visceral hypersensitivity
This refers to an exaggerated response to normal visceral stimuli, mediated by central and peripheral sensitization. It is common in IBS, FD, FC, and functional abdominal bloating8. Hyperalgesia may involve different digestive regions; for example, patients with FD often present with gastric hypersensitivity, whereas rectal hypersensitivity predominates in IBS9,10.
Motility disorders
These may affect various segments of the GI tract, manifesting as delayed gastric emptying (in IBS and FD), slow colonic transit (in IBS with constipation [IBS-C] and FC), or hypocontractility. Such disorders may influence each other through reflex and neurohormonal mechanisms, such as interference of colonic transit with gastric accommodation7. In addition, fermentation products such as short-chain fatty acids may modulate motility and sphincter tone, exacerbating symptoms of GERD or bloating5.
Intestinal dysbiosis
An imbalance of the luminal and mucosa-associated microbiota may impair the epithelial barrier, induce subclinical inflammation, and modify neuroimmune signaling of the gut-brain axis (GBA)7,11. These alterations are also associated with systemic manifestations such as fatigue, anxiety, or sleep disorders, particularly in patients with multiple DGBI or comorbidities such as FM12,13.
GBA dysfunction
The GBA is a bidirectional network integrating the central nervous system, the enteric nervous system, the immune system, and the intestinal microbiota8. Its disruption may amplify pain perception, impair emotional regulation, and modulate autonomic responses, explaining the multisystemic expression of many DGBI7,13 (Fig. 2).
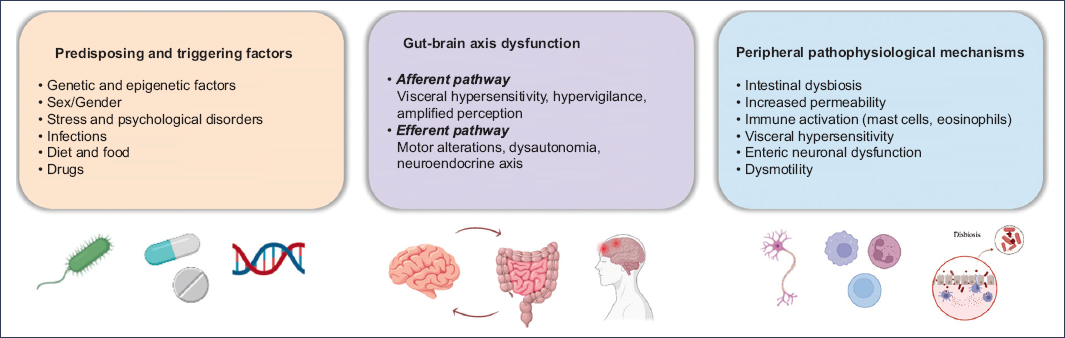
Figure 2. Pathophysiologic model of disorders of gut-brain interaction.
Clinical course and symptom fluctuation
Overlap among DGBI leads to a more protracted clinical course, characterized by more persistent and longer-lasting symptoms, greater variability over time, reduced response to conventional therapies, and increased use of diagnostic and health care resources.
Moreover, the coexistence of multiple DGBI favors subtype fluctuation. In IBS, only 24% of patients maintain the same subtype over time, while more than 50% shift between IBS-C, IBS with diarrhea [IBS-D], and mixed IBS14. This symptomatic instability requires periodic clinical reassessment and a flexible therapeutic approach adapted to each patient’s clinical course.
From the outset, the possibility of overlap should be considered. This involves a targeted history exploring symptoms in different anatomic regions, assessment of psychosocial comorbidity, identification of the predominant symptomatic axis, and prioritization of the most disabling symptom as a therapeutic guide.
Recognizing these patterns helps avoid fragmented treatments, reduce unnecessary interventions, and promote an integrated perspective aimed at improving patient quality of life.
Impact on the patients’ quality of life
Overlap of IBS with other DGBI and extra-digestive comorbidity negatively affects multiple dimensions of quality of life, including physical, emotional, functional, social, and occupational aspects. This impact extends beyond digestive discomfort, affecting the patient’s overall experience.
Several studies have shown that patients with multiple DGBI present with greater symptom severity, higher somatic burden, health-related anxiety, and more significant interference with daily activities. They also tend to use health services more frequently and report lower therapeutic satisfaction15–18.
The number of DGBI correlates directly with poorer general health perception, greater work absenteeism, and reduced productivity. In the Rome Global Study19, individuals with symptoms in multiple anatomic regions reported significantly lower scores across all SF-36 domains, particularly in physical, emotional, and functional dimensions.
Furthermore, the coexistence of visceral symptoms with somatization or psychological disorders increases the likelihood of meeting diagnostic criteria for mental disorders according to the Diagnostic and Statistical Manual of Mental Disorders. This fosters a negative feedback loop in which physical symptoms amplify anxiety and catastrophization, which in turn intensify pain perception7,20,21.
Many patients also report feeling stigmatized or invalidated by medical personnel, which may lead to frustration, repeated health care seeking, unnecessary interventions, and poor treatment adherence. Lack of recognition of the complexity of their clinical presentation contributes to this perception.
For all these reasons, quality of life should be considered an explicit therapeutic goal. The approach must be integrative and patient-centered, including:
- – Symptom relief directed at the predominant axis.
- – Psychosocial interventions (eg, cognitive-behavioral therapy [CBT] or mindfulness).
- – Empathetic validation of the patient’s experience.
- – Education about the chronic and multifactorial nature of the disorder.
- – An interdisciplinary strategy with realistic goals, which may substantially improve health perception and reduce overall disease burden2,22.
Diagnostic and therapeutic implications of IBS overlap
Recognition of shared pathophysiologic mechanisms among DGBI has important clinical implications. In practice, it is common for patients to present symptoms in more than one anatomic region or to meet criteria for multiple DGBI, which demands a more flexible and integrative diagnostic approach.
Diagnosis in the context of overlap
Although the Rome IV criteria are useful for classifying DGBI, their strict application may limit the identification of overlapping conditions. For example, a significant proportion of patients with FD also meet criteria for IBS, particularly in the subtypes of postprandial distress syndrome and epigastric pain syndrome, with overlap prevalence ranging from 17% to 55%7.
A diagnostic approach should focus on:
- – Detailed history exploring symptoms in different anatomic regions and their relationship with diet, stress, or defecation.
- – Systematic evaluation of psychological or somatic comorbidity.
- – Use of validated tools such as questionnaires or pictograms to facilitate communication between physician and patient.
- – Identification of the predominant or most disabling symptom as the therapeutic target.
Studies have shown that a clinical diagnosis based on these elements is usually stable over time, with a low risk of missing relevant organic disease if the initial evaluation is adequate.
Clinical course and symptom fluctuation
As noted previously, DGBI are dynamic disorders with high temporal variability. In IBS, more than 50% of patients modify their subtype during follow-up. This instability requires periodic reassessment and individualized therapeutic adjustments according to symptom progression14.
Therapeutic implications
Treatment should target the dominant pathophysiology and the most disabling symptomatic axis. Rigid or purely symptom-based approaches should be avoided. In many cases, a rational combination of therapies is more effective. For example, treating constipation in patients with IBS-C may also improve postprandial fullness in cases overlapping with postprandial distress syndrome.
The use of neuromodulators—such as tricyclic antidepressants, selective serotonin reuptake inhibitors (SSRIs), serotonin–norepinephrine reuptake inhibitors (SNRIs), or calcium-channel ligands—has shown efficacy in the management of pain, dysmotility, anxiety, and visceral hypervigilance. Recommendations include:
- – Avoid using terms such as antidepressant or antipsychotic to reduce stigma and improve adherence.
- – Start with low doses and adjust progressively.
- – Avoid combining agents from the same class to reduce the risk of adverse effects, such as serotonin syndrome.
Additionally, some neuromodulators have proven beneficial in frequent comorbidities such as FM, migraine, or interstitial cystitis.
Role of mental health
Identifying affective symptoms is key to an effective therapeutic strategy. Referral to psychiatry or psychology is recommended in cases of moderate-to-severe affective disorders and in patients with persistent somatic symptoms. Non-pharmacologic therapies (eg, CBT, hypnotherapy, or mindfulness) should also be considered as part of an integrative treatment plan2.
Overlap between IBS and other disorders of gut-brain interaction
IBS and FD
FD is the condition most frequently overlapping with IBS. Up to 64% of patients with FD also meet criteria for IBS, particularly IBS-D and postprandial distress syndrome23,24. This combination is associated with greater symptom severity, higher emotional burden, and poorer quality of life.
In a Pan-Asian survey25, between 25% and 33% of IBS patients reported epigastric pain as their predominant symptom, forming an independent symptom cluster.
Both conditions share several pathophysiologic mechanisms, such as visceral hypersensitivity, duodenal dysfunction (with increased mast cells and eosinophils), dysbiosis, and an exaggerated gastrocolic reflex. Overlap may also occur as a post-infectious sequela after acute gastroenteritis.
Treatment should target the predominant symptom, and strategies include:
- – Visceral neuromodulators (eg, amitriptyline, duloxetine).
- – H1/H2 antihistamines in patients with allergic features or postprandial hypersensitivity.
- – Prokinetics in cases with associated dysmotility.
- – Low-FODMAP diet.
- – Diaphragmatic relaxation techniques and psychosocial interventions.
IBS and FC
Differentiating IBS-C from FC can be challenging, as they share symptoms such as infrequent bowel movements, straining, excessive effort, and incomplete evacuation. The presence of recurrent abdominal pain supports IBS-C, whereas its absence favors FC.
Studies based on Rome III criteria have reported that 18-44% of patients meet criteria for both disorders26,27, reflecting significant symptomatic overlap. Furthermore, the Rome Global Epidemiology Study found that nearly one-third of patients initially diagnosed with IBS-C or FC migrated to the other diagnosis over time, highlighting their dynamic nature23.
From a pathophysiologic standpoint, slow colonic transit and pelvic floor dyssynergia are more frequent in FC, whereas visceral hypersensitivity predominates in IBS-C. However, these findings are not mutually exclusive: defecatory dyssynergia has been documented in 59% of FC patients but also in a relevant proportion of IBS-C patients. Similarly, slow transit is observed in 47% of FC and 22-30% of IBS-C patients28.
Clinically, IBS-C/FC overlap is associated with poorer quality of life, higher anxiety and depression rates, and more frequent medical visits. These differences also affect treatment response. While secretagogues and prokinetics may benefit both groups, antidepressants and CBT appear more effective in IBS-C, whereas pelvic floor biofeedback is key for dyssynergia, typical of some FC cases29.
Overall, IBS-C and FC should be regarded as related conditions within a shared clinical and pathophysiologic spectrum. Management should be individualized according to the presence or absence of pain, transit pattern, available physiologic testing, and the patient’s psychological profile.
IBS and GERD
IBS and GERD overlap in up to 47% of cases, according to observational studies23. This coexistence is not only common but also clinically significant, as it is associated with greater refractoriness to conventional therapy and worse quality of life.
Both disorders share pathophysiologic mechanisms such as visceral hypersensitivity, segmental dysmotility, and psychosocial vulnerability10. Manometric studies have shown that colonic distension may induce transient lower esophageal sphincter relaxations, exacerbating GERD symptoms30, suggesting a reflex interaction between different segments of the digestive tract.
Moreover, patients with IBS and GERD have higher prevalence of anxiety, somatic hypervigilance, and catastrophization, which amplifies symptom perception.
Treatment should address global GBA dysfunction, and strategies include:
- – Neuromodulators such as duloxetine or low-dose tricyclic antidepressants.
- – CBT or emotional regulation therapies.
- – Specific dietary adjustments (eg, avoiding large meals and trigger foods).
- – Optimization of antisecretory therapy in cases of erosive GERD.
It is essential to avoid unnecessary escalation of proton pump inhibitor therapy when symptoms persist despite endoscopic normalization and to consider functional mechanisms associated with IBS.
IBS and functional abdominal bloating
Abdominal bloating is a highly prevalent symptom in IBS patients, particularly those with IBS-C; it is estimated that up to 90% of IBS patients experience it at some point31. In many cases, visible distension is not related to objective gas accumulation but rather to motor disturbances, diaphragmatic dysfunction, or abnormal somatic perception.
Studies using abdominal plethysmography and magnetic resonance imaging have shown that functional distension may result from a dyssynergic breathing pattern, with paradoxical diaphragmatic descent during inspiration and secondary abdominal protrusion. This alteration may be unconscious and reflect a component of somatization or visceral hypervigilance.
From a pathophysiologic standpoint, impaired intestinal accommodation to normal volumes of gas or luminal contents has also been proposed, exacerbated by segmental dysmotility and alterations in the microbiota.
Therapeutic approaches include:
- – Diaphragmatic breathing training with abdominal biofeedback.
- – Postural and breathing pattern re-education therapies.
- – Neuromodulators in cases with predominant visceral hypersensitivity.
- – Psychosocial interventions to reduce excessive somatic attention (eg, CBT or mindfulness)
Patient education regarding the functional pathophysiology of bloating and validation of their experience are essential to improve adherence and reduce symptom-related anxiety.
Extra-digestive comorbidity
IBS and IBD
Although IBD and IBS are distinct entities, up to 30-40% of patients with IBD in clinical remission present IBS-like symptoms. This situation is more frequent in ulcerative colitis and represents a significant diagnostic and therapeutic challenge7,32.
In these cases, functional symptoms may be due to persistent alterations of the intestinal mucosa, even in the absence of evident active inflammation. Frequent findings include:
- – Visceral hypersensitivity assessed by barostat, associated with higher mast cell density and overexpression of receptors such as TRPV1.
- – Subclinical inflammation, with elevated fecal calprotectin, intraepithelial lymphocyte infiltration, and increased proinflammatory cytokines (eg, TNF-α), despite apparent clinical and endoscopic remission33.
- – Post-inflammatory neuroimmune changes that perpetuate visceral sensitization through persistent plasticity in afferent pathways32,34
Clinically, it is crucial to distinguish functional symptoms from an inflammatory flare. Biomarkers (eg, fecal calprotectin), imaging, and, when necessary, endoscopy are recommended.
Recognition of IBS–IBD overlap prevents unnecessary use of immunosuppressants or biologics and allows management to focus on strategies targeting functional symptoms, such as:
- – Low-FODMAP diet.
- – Intestinal neuromodulators.
- – Psychosocial approaches such as CBT or hypnotherapy
This integrative strategy improves symptom control and reduces both the emotional and medical burden of patients35.
IBS and urogynecologic disorders
Women with IBS frequently present concomitant pelvic dysfunctions, particularly dysmenorrhea, dyspareunia, chronic pelvic pain, and overactive bladder. This coexistence reflects shared pathophysiologic mechanisms, such as viscerovisceral hypersensitivity and sensory convergence at the spinal level.
It has been proposed that bladder or gynecologic irritation may sensitize shared afferents, exacerbating digestive symptoms. Additionally, factors such as dysbiosis, low-grade inflammation, and autonomic dysfunction may modulate both pain perception and pelvic motility.
Evaluation of these cases should include a detailed history with a multidisciplinary focus. Therapeutic management benefits from collaboration among gastroenterology, gynecology, urology, pelvic floor physiotherapy, and mental health. Recommended strategies include:
- – Pelvic floor rehabilitation exercises and biofeedback.
- – Neuromodulators with visceral and somatic action (eg, amitriptyline, duloxetine).
- – Psychotherapeutic interventions addressing hypervigilance, anxiety, or chronic pain.
- – Specific treatments for urinary or gynecologic dysfunctions (per specialist assessment)
Symptom validation, integrative management, and interdisciplinary coordination are key to improving clinical outcomes and quality of life in this population7.
INTERSTITIAL CYSTITIS OR BLADDER PAIN SYNDROME
Interstitial cystitis or bladder pain syndrome (IC/BPS) is characterized by chronic bladder pain in the absence of demonstrable urinary infection. Its prevalence in IBS patients is estimated at 30-40%36, reflecting a clinically significant association.
Both conditions share similar pathophysiologic mechanisms, such as cross-sensitization between intestinal and bladder visceral afferents, chronic neuroinflammation, and autonomic dysfunction. Increased mast cells in the bladder mucosa and enhanced local histamine release—also described in the intestinal mucosa of IBS patients—have been observed1,36.
Clinically, IBS-IC/BPS overlap presents with greater pain intensity, persistent urinary symptoms, abdominal discomfort, and a marked impact on quality of life, particularly in young women.
Management must be multidisciplinary and personalized, including:
- – Neuromodulators addressing both visceral and somatic pain (eg, duloxetine, pregabalin).
- – Psychological therapies focused on chronic pain and anticipatory anxiety.
- – Pelvic physiotherapy to improve tone and muscular relaxation.
- – Local measures in selected cases (eg, bladder instillations, antihistamines, topical analgesics)
Timely diagnosis and coordinated treatment are essential to prevent chronification and improve functional outcomes.
DYSMENORRHEA AND CHRONIC PELVIC PAIN
Between 20% and 50% of women with IBS report dysmenorrhea, and up to 35% experience chronic pelvic pain. This coexistence has been attributed to central and peripheral sensitization mechanisms, as well as viscerovisceral convergence phenomena that amplify pain perception7.
Cyclic gynecologic pain may potentiate intestinal hyperalgesia and vice versa, generating a cross-feedback phenomenon between pelvic organs. Dysmenorrhea and chronic pelvic pain are also associated with higher prevalence of anxiety, sleep disorders, and autonomic dysfunction—all of which may exacerbate IBS.
Differential diagnosis with other causes of pelvic pain (eg, endometriosis, IC/BPS, uterine fibroids) is essential and requires appropriate gynecologic evaluation.
Management must be integrative and individualized, including:
- – Dual neuromodulators effective against both visceral and somatic pain.
- – Hormonal interventions when a predominant gynecologic etiology is suspected.
- – CBT and stress management.
- – Pelvic floor physiotherapy in cases of associated muscular dysfunction
Symptom validation and interdisciplinary coordination are fundamental to prevent excessive medicalization, reduce functional impact, and improve quality of life in these patients.
ENDOMETRIOSIS
Endometriosis is a chronic inflammatory disease that affects approximately 10% of women of reproductive age and is frequently associated with GI symptoms that mimic or coexist with IBS. The overlap of both conditions is common and clinically relevant. Studies have reported that between 30% and 50% of women with endometriosis meet diagnostic criteria for IBS37.
Even after surgical treatment of endometriosis, many patients continue to experience IBS-compatible symptoms, reflecting the persistence of underlying functional mechanisms. Shared pathophysiologic factors include:
- – Chronic low-grade inflammation.
- – Visceral hypersensitivity.
- – Intestinal dysbiosis.
- – Persistent central and peripheral sensitization.
The coexistence of endometriosis and IBS is associated with greater symptom severity, impaired quality of life, increased medical consultations, and more frequent use of empirical treatments before achieving a definitive diagnosis.
Management should include:
- – Systematic evaluation of gynecologic symptoms in patients with IBS.
- – Close coordination with gynecology in complex or refractory cases.
- – Rational use of neuromodulators with both visceral and somatic effects.
- – Consideration of nonpharmacologic therapies such as pelvic floor physiotherapy, CBT, and chronic pain management strategies.
Since many women with endometriosis initially present to the gastroenterologist, it is essential to maintain a high index of suspicion, particularly in young patients with chronic abdominal pain, changes in bowel habits, and concurrent cyclic or gynecologic symptoms.
Fibromyalgia (FM) and chronic fatigue syndrome (CFS)
FM and CFS are frequent comorbidities in patients with IBS. Up to 65% of IBS patients meet criteria for FM or CFS, while approximately 70% of FM patients report IBS-like symptoms.
These conditions share pathophysiologic mechanisms:
- – Central and peripheral sensitization.
- – Dysfunction of the GBA and the autonomic nervous system.
- – Visceral and somatic hypersensitivity.
- – Low-grade neuroinflammation.
- – Immune alterations and intestinal microbiota disturbances
Furthermore, they are associated with sleep disorders, somatic hyperalertness, hypervigilance, catastrophizing, and avoidance behaviors, all of which contribute to symptom chronicity and severity.
Patients with IBS and FM or CFS present greater functional disability, worse quality of life, higher rates of absenteeism, and increased healthcare utilization. Some studies have shown that these patients seek medical attention up to four times more often than those without comorbidity and have a poorer response to conventional treatment12,38.
Similar dysbiosis patterns have also been identified in patients with IBS and FM, suggesting a possible common pathway of alteration in the microbiota-gut-brain axis.
Therapeutic management should be multimodal and include:
- – Dual neuromodulators such as duloxetine, pregabalin, or tricyclic antidepressants.
- – Stress regulation techniques and chronic pain education.
- – Cognitive and psychoeducational therapies.
- – Lifestyle interventions, including sleep hygiene, gradual physical activity, and functional nutrition.
Treatment should focus on improving functionality and quality of life rather than complete symptom elimination, reinforcing patient autonomy and avoiding excessive interventions.
Conclusions
The overlap of IBS with other DGBIs and systemic comorbidities is a frequent, complex, and highly relevant clinical phenomenon. Recognition of this overlap implies a paradigm shift: moving away from addressing symptoms in isolation toward an integrated, patient-centered perspective based on shared underlying mechanisms.
The coexistence of multiple DGBIs and comorbidities such as FM, endometriosis, or IC/BPS not only increases symptom burden and worsens quality of life but also requires more careful evaluation and individualized therapeutic strategies. Shared mechanisms—including visceral hypersensitivity, gut–brain axis dysfunction, neuroinflammation, and intestinal dysbiosis—justify a common pathophysiologic approach.
From a diagnostic standpoint, it is essential to perform a targeted history, identify the predominant symptom axis, and consider the dynamic evolution of subtypes. Therapeutically, rational use of neuromodulators, psychotherapeutic interventions, lifestyle-based therapies, and patient validation are crucial to improve clinical outcomes.
Ultimately, successful management of these patients requires empathy, a deep understanding of the DGBI spectrum, and a collaborative interdisciplinary approach. Integrating these elements will enable more human, effective, and evidence-based care.
Acknowledgments
The author thanks Dr. José María Remes-Troche for his kind invitation to contribute to this special issue of Clínicas de Gastroenterología de México, as well as the Mexican Association of Gastroenterology for its commitment to continuing medical education and dissemination of knowledge in the field of gastroenterology.
Funding
The author declared that no funding was received for this study.
Conflicts of interest
The author declared no conflicts of interest. whatsoever.
Ethical considerations
Protection of humans and animals. The author declares that no experiments were conducted on humans or animals for this research.
Confidentiality, informed consent, and ethics approval. The study does not involve patient data and did not require ethics approval. The SAGER guidelines do not apply.
Declaration on the use of artificial intelligence. The author declares that no generative artificial intelligence tools were used in the preparation of this manuscript.
