Definition
Irritable bowel syndrome (IBS) is a disorder of gut–brain interaction characterized by abdominal pain associated with changes in bowel habits, and it can be categorized into 4 subtypes depending on the predominant bowel pattern: IBS with diarrhea predominance (IBS-D), constipation predominance (IBS-C), mixed (IBS-M), or unclassified (IBS-U)1. IBS-D is defined, according to the Rome IV diagnostic criteria, by the presence of recurrent abdominal pain occurring at least once per week during the past 3 months, with symptom onset at least 6 months prior to diagnosis, associated with diarrhea predominance, defined as decreased stool consistency (Bristol types 6 or 7) or increased stool frequency during > 25% of total bowel movements, and hard stools (Bristol 1 or 2) in < 25% of bowel movements1–3. It differs from functional diarrhea (FD) in that the cardinal symptom in IBS-D is pain1.
Epidemiology
The incidence rate of IBS is estimated at 1.3-1.5%1, while prevalence varies according to geographic region and diagnostic criteria used. The global prevalence, according to the Rome group, ranges from 7% to 21%4,5. Using Rome I criteria, prevalence is 8.8%; Rome II, 7.8-9.4%; Rome III, 9.1-11.5%; and Rome IV, 5-10%5,6. In Mexico, prevalence varies by region and is estimated between 4% and 35%7–11. Data on prevalence by subtype are more limited, but IBS-D, IBS-C, and IBS-M appear to occur at similar rates. Some reports indicate IBS-D is more common, accounting for up to 40% of cases12,13, whereas in Mexico it represents a smaller proportion (9%)10,14.
Pathophysiology of irritable bowel syndrome
IBS-D is a multifactorial disorder involving multiple coexisting pathophysiological mechanisms, including genetic predisposition, alterations of the intestinal barrier, permeability and low-grade inflammation, food allergies and intolerances, bile acid–associated diarrhea (BAD), dysbiosis, neuroimmune dysfunction, abnormal signaling, visceral hypersensitivity, and dysregulation of the brain-gut axis with central and peripheral pain-processing alterations2,15–20. A common risk factor for IBS-D is a history of infectious gastroenteritis or food poisoning, which increases the risk of developing postinfectious IBS (PI-IBS) 4-fold, with a cumulative prevalence of 7.3-10.1%, reaching 15-30% during epidemics or 5-10% after traveler’s diarrhea21,22. Another recently involved factor is severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, particularly in patients with gastrointestinal manifestations during the acute phase23.
Evaluation and differential diagnosis
The main differential diagnoses of IBS-D include other causes of abdominal pain associated with chronic diarrhea, such as postoperative anatomic/physiological changes (cholecystectomy, gastrojejunal bypass, intestinal resections, colectomy), adverse drug effects (laxatives, secretagogues, enterokinetics, antibiotics, gastric antisecretory agents, angiotensin inhibitors, levothyroxine, orlistat, acarbose, selective serotonin reuptake inhibitors [SSRIs], colchicine), infectious causes (Giardia, Clostridioides difficile), inflammatory causes (inflammatory bowel disease [IBD], microscopic colitis), and malabsorptive causes (small intestinal bacterial overgrowth [SIBO], celiac disease and other enteropathies, exocrine pancreatic insufficiency)24. National and international clinical practice guidelines propose different diagnostic strategies (Fig. 1)13,24–27. Most recommend ruling out infectious causes, particularly Giardia, via stool studies such as a fecal GI PCR panel, and ruling out IBD initially using noninvasive fecal biomarkers such as quantitative calprotectin. Malabsorption syndromes should be evaluated with a complete blood count, nutrient absorption markers (serum vitamin B12, folic acid, iron studies), and celiac serology (IgA anti-tissue transglutaminase, total serum IgA, anti-endomysial antibodies, or deamidated anti-gliadin IgG). In the presence of alarming features, endoscopic studies with biopsies are indicated to exclude microscopic and eosinophilic enterocolitis. In patients with recent gastroenteritis and persistent symptoms, breath tests may be used to confirm or rule out SIBO, or antibodies vs Campylobacter toxin (anti-CdtB) and antivinculin may be tested in suspected PI-IBS, although sensitivity is low even with newer epitope corrections. Finally, BAD should be excluded, though due to limited availability of assays, it is often diagnosed indirectly with a therapeutic trial of bile acid sequestrants13,24–26.
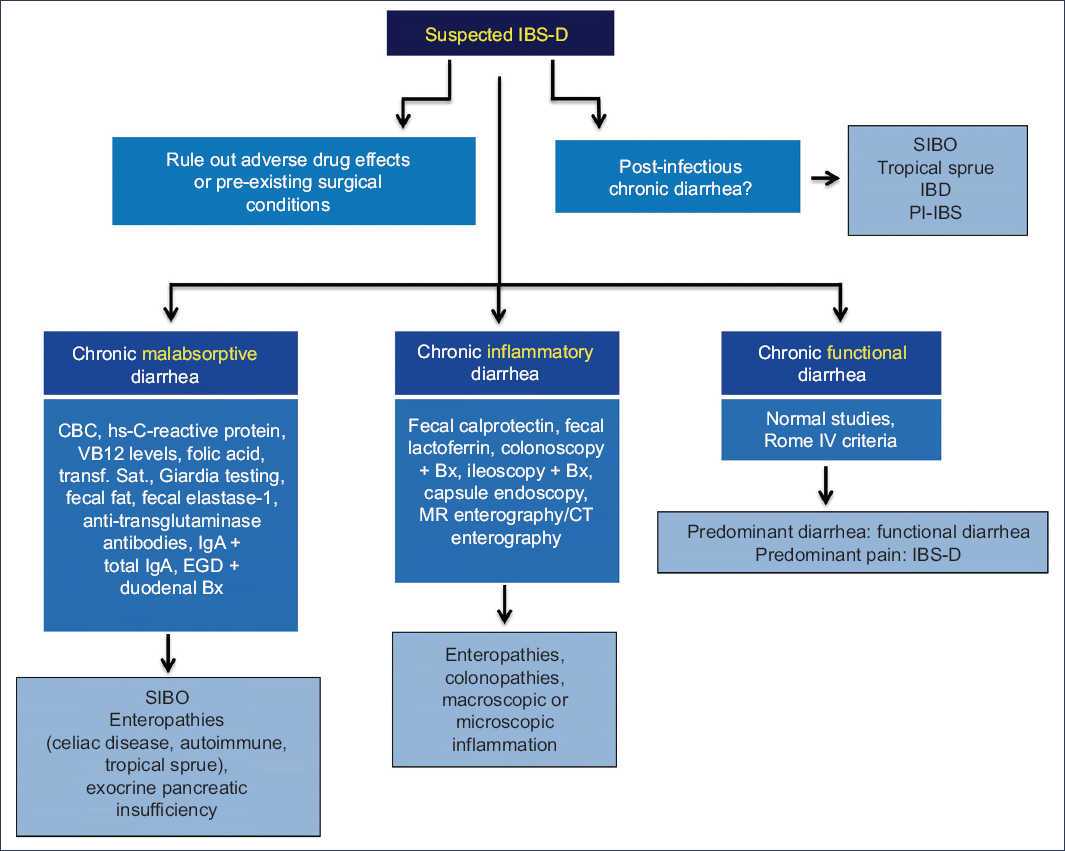
Figure 1. Diagnostic approach for IBS-D/functional diarrhea. SIBO, small intestinal bacterial overgrowth; IBD, inflammatory bowel disease; IBS-D, irritable bowel syndrome with diarrhea; PI-IBS, postinfectious irritable bowel syndrome; CBC, complete blood count; hsCRP, high-sensitivity C-reactive protein; VB12, vitamin B12; transf. sat, transferrin saturation; EGD, esophagogastroduodenoscopy; Bx, biopsy; MRI, magnetic resonance imaging; CT, contrast-enhanced computed tomography.
Treatment
A better understanding of IBS-D and FD pathophysiology has enabled the evaluation of targeted therapies (Table 1) including dietary interventions, motor-altering therapies (antispasmodics), treatments aimed at low-grade inflammation or intestinal permeability (mesalamine, steroids, ebastine, larazotide), therapies targeting SIBO or dysbiosis (rifaximin, probiotics, prebiotics, fecal microbiota transplant), drugs modulating fluid homeostasis (loperamide, eluxadoline, alosetron, ramosetron, ondansetron, lidamidine), bile acid malabsorption therapies (ion-exchange resins: cholestyramine, colestipol, colesevelam), and neuromodulators15,18,28–30.
Table 1. Treatment of irritable bowel syndrome with diarrhea
| Pathophysiological mechanism | Therapeutic options | Medical evidence |
|---|---|---|
| Dietary/food intolerance | Low-FODMAP diet Gluten-free diet | Symptomatic improvement, individualized |
| Motor alterations | Antispasmodics | Pain improvement in short/medium term, NNT 3–11 |
| Low-grade inflammation | Mesalamine, prednisolone, ketotifen, cromoglycate, ebastine | Inflammatory score improvement, no symptomatic benefit |
| Dysbiosis | Locally acting antibiotics (rifaximin) Probiotics | Global improvement: stool number and consistency, bloating, SIBO, IBS-non-C Symptom improvement overall |
| Intestinal permeability alterations | Glutamine, larazotide | Pilot study, no controlled trials |
| Fluid homeostasis alterations | 5-HT3antagonists (alosetron, ondansetron, ibodutant) | Improvement in stool number and consistency (A, O); antinociceptive effect (A); NNT 4–8 (A) Antinociceptive (in women) |
| Bile acid homeostasis alterations | Cholestyramine, colesevelam, colestipol Eluxadoline Liraglutide | Indirect evidence (bile acid diarrhea) Symptomatic improvement (in healthy gallbladder) Not inferior to colesevelam |
| Brain–gut axis dysfunction | Neuromodulators: tricyclics (amitriptyline, imipramine, nortriptyline) SSRIs, SNRIs | Global improvement, pain score, NNT 3 Global improvement as a group |
|
5-HT3, 5-hydroxytryptamine; A, alosetron; FODMAP, fermentable oligosaccharides, disaccharides, monosaccharides, and polyols; SNRI, serotonin–norepinephrine reuptake inhibitors; SSRI, selective serotonin reuptake inhibitors; NNT, number needed to treat; O, ondansetron; SIBO, small intestinal bacterial overgrowth; IBS-non-C, irritable bowel syndrome without constipation; GB, gallbladder. |
||
Therapies targeting food intolerance or food-related symptoms
Although most patients associate diet as a symptomatic trigger, only 11-27% have documented intolerance to a specific diet. Diet can cause symptoms through osmotic, chemical, or mechanical effects, or by modifying the microbiota. To date, there is no standardized nutritional protocol useful for all patients with IBS-D, so diet should be individualized according to history, tolerance, and the particular response of each patient. Some general measures, such as moderating portions, reducing insoluble fiber, avoiding sorbitol and artificial sweeteners, caffeine, fructose, and alcohol, may be useful for some patients. The low-FODMAP diet (acronym for fermentable oligosaccharides, disaccharides, monosaccharides, and polyols) allows the identification of foods commonly triggering pain and bloating, and can be individualized after an initial identification phase. Different protocols exist for long-term implementation. A gluten-free diet has not been shown to improve IBS-D symptoms unless the patient has celiac disease, although it is important to consider that there may be overlap between both entities28,31,32.
Therapies targeting motor alterations and symptomatic management
Antispasmodics are drugs that relax intestinal smooth muscle through calcium antagonist effects, by blocking sodium channels, or through antimuscarinic/anticholinergic effects, and even via more than one receptor, including 5-HT (5-hydroxytryptamine) and neurokinin. They are categorized into several groups: calcium antagonists (pinaverium bromide, alverine citrate, fenoverine, rociverine, pirenzepine, and peppermint oil); tertiary amines (trimebutine and mebeverine); quaternary ammonium derivatives (pinaverium bromide, otilonium bromide, and prifinium bromide); scopolamine derivatives (butylscopolamine, hyoscyamine, dicyclomine, and cimetropium); and phenol derivatives (phloroglucinol)33. Antispasmodics are the first-line therapy for short- and mid-term symptomatic control of abdominal pain. Several meta-analyses have demonstrated their superiority over placebo for improvement of global symptoms (number needed to treat [NNT] = 5) and abdominal pain scores (NNT = 7, range 3-11). Although evidence is more limited, the individual NNTs for each antispasmodic are similar: otilonium bromide and mebeverine NNT = 5; pinaverium bromide, alverine, and dicyclomine NNT = 4; hyoscine bromide and cimetropium NNT = 311,27,33–37. Several commercial preparations combine antispasmodics with simethicone/dimethicone (an antifoaming agent that decreases surface tension of mucogaseous bubbles) and/or galactosidase (an enzyme that hydrolyzes non-absorbable oligosaccharides), which seem to provide additional benefit for bloating. Some antispasmodics, due to their anticholinergic activity, may induce constipation and thereby improve bowel habits in IBS-D patients; however, no studies have specifically evaluated this effect11,34,36.
Therapies targeting low-grade inflammation
Several lines of research have evaluated drugs aimed at reducing low-grade intestinal inflammation, including locally acting steroids (e.g., budesonide), systemic corticosteroids (prednisolone), mesalazine, mast cell stabilizers (ketotifen and cromoglycate), and histamine antagonists (ebastine). Current evidence with prednisolone and cromoglycate, and prior evidence with mesalazine, indicates that although they improve inflammation scores (decreased inflammatory cell counts), this improvement does not translate into significant clinical benefit28,30,38. However, a recent systematic review and meta-analysis including eight studies (n = 820) concluded that mesalazine may be modestly effective in improving global symptoms, particularly in IBS-D, but not in other subgroups or in post-infectious IBS (relative risk [RR], 0.88; 95% confidence interval [CI], 0.79-0.99), although the quality of evidence is low39. Preliminary evidence has reported that both ketotifen and ebastine increase rectal sensory thresholds to barostat distension in a subgroup of IBS patients, but they have not shown true utility in IBS-D29,30. It has been proposed that drugs aimed at restoring intestinal permeability could improve symptoms in IBS-D. From a hypothetical and experimental perspective, glutamine and larazotide acetate may affect claudin-1 expression (a protein associated with permeability) and regulate tight junctions, respectively. However, no controlled studies exist in IBS or IBS-D30.
Therapies targeting dysbiosis
Multiple studies have demonstrated a central role of the microbiota in the pathophysiology of IBS, and alterations such as small intestinal bacterial overgrowth (SIBO) or dysbiosis may be associated with symptom development. Several options aimed at treating SIBO (local-acting antibiotics such as rifaximin) and dysbiosis (probiotics, prebiotics, and fecal microbiota transplantation) have been tested in IBS40,41. Rifaximin is a broad-spectrum, non-absorbable synthetic antibiotic that inhibits bacterial RNA synthesis, with five polymorphs (α, β, γ, δ, and ε). It is approved for use in SIBO and non-constipation IBS (IBS-nC)42 based on results from the TARGET 1 and 2 trials, where a 14-day regimen of rifaximin-α at 550 mg 3 times daily was associated with significant improvement in global symptoms (primary endpoint), and in pain, bloating, and stool consistency (loose or watery) (secondary endpoints)43. The responder definition in the diarrhea sub-analysis was ≥ 50% reduction in baseline days with Bristol type 6 or 7 stools during at least 2 of the first 4 weeks of treatment43. Subsequent studies from the same cohort, such as TARGET-3, demonstrated improvement with repeated treatments, prediction of response when SIBO was confirmed by breath test, and a sub-analysis also reported abdominal pain improvement in 56.8% of patients44,45. Proposed mechanisms of action behind rifaximin’s effects include elimination of pathogenic bacteria, modification of microbiota composition and diversity, altered expression of virulence factors, and indirect effects from dysbiosis correction such as anti-inflammatory or barrier-protective activity42.
Probiotics are microorganisms that, when administered in adequate amounts, provide beneficial effects to the host, either directly through microbial diversity or indirectly via immune stimulation and mucosal function improvement. Several systematic reviews and meta-analyses have shown a limited but significantly superior effect of probiotics as a group vs placebo in improving global symptoms, with a significant reduction in persistence of symptoms (RR, 0.79; 95% CI, 0.68-0.91) and NNT = 7, with stronger evidence for combinations than for single strains46. Sub-analyses have reported improvements in pain and flatulence scores, and a trend toward improvement in bloating, but heterogeneity of studies limits findings, and evidence of benefit by IBS subtype is scarce40,41.
Fecal microbiota transplantation is an emerging therapy for conditions associated with severe dysbiosis such as recurrent C. difficile infection. In IBS, it has been evaluated in several clinical trials with conflicting results. A recent meta-analysis of eight randomized controlled trials did not demonstrate significant benefit in symptoms at 3 months post-treatment47.
Therapies targeting altered fluid and bile acid homeostasis
Four groups of drugs may improve IBS-D symptoms by addressing different mechanisms involved in increased peristalsis, secretion, and motility: opioid agonists (loperamide and eluxadoline), 5-HT3 antagonists (alosetron, ramosetron, and ondansetron), α2 agonists (lidamidine), and bile acid sequestrants (cholestyramine, colestipol, colesevelam, and obeticholic acid).
Opioid receptor agonists (loperamide and diphenoxylate) increase intestinal fluid absorption and reduce GI and colonic transit and are frequently used as antidiarrheal agents. Loperamide has been evaluated in clinical studies using older IBS-D criteria and was shown to be superior to placebo for global symptom improvement, stool consistency, and abdominal pain, although evidence quality is low48. Eluxadoline is a drug with dual effects on opioid receptors: it is an agonist at μ- and κ-receptors, and an agonist at the σ-receptor. A pivotal study including 2,427 patients compared 2 doses (75 and 100 mg) vs placebo, with the primary endpoint being the proportion of patients with a reduction in the composite pain score and improved stool consistency on the same day in ≥ 50% of days across 12 and 26 weeks. Both eluxadoline doses achieved significantly superior results compared to placebo49. The NNT to achieve these endpoints was 850. Additional studies demonstrated its effectiveness in different outcomes and subgroups: effective in patients with either adequate or inadequate prior response to loperamide51, similar efficacy to antispasmodics according to a recent network meta-analysis (though with a higher rate of side effects)52, and sustained benefits up to 6 months when an initial response was achieved53. Reported adverse events include increased risk of pancreatitis and sphincter of Oddi spasm in cholecystectomized patients, so its use is recommended only in those with an intact gallbladder54,55.
5-HT is an important modulator of intestinal secretion and nociception. Several 5-HT3 receptor antagonists have been studied in IBS-D (alosetron, ramosetron, ondansetron). Alosetron, the most extensively studied, has a therapeutic gain of 20–30% over placebo and an NNT of 4-8 (0.5 mg twice daily) for reducing stool frequency, in addition to antinociceptive effects and improved quality of life. However, its safety profile is associated with severe constipation and ischemic colitis, and in the United States it is available only under a restricted prescription program56,57. Ramosetron was evaluated in four RCTs, and a recent meta-analysis including 1,623 patients reported improvements in global symptoms, abdominal pain/discomfort, bowel habits, and stool consistency58. Neither alosetron nor ramosetron are available in Mexico. Ondansetron, available in Mexico, was evaluated in an RCT with titrated doses (4-8 mg/day), initially designed for 400 patients but interrupted by the 2020 pandemic after 80 were randomized; compared to placebo, it showed a 12% therapeutic gain. Pooling this trial with prior studies yields an NNT of 5 for stool consistency improvement, and 9 for achieving the FDA composite endpoint (global improvement, pain, and stool consistency), but without significant effect on abdominal pain59–61.
Lidamidine, an α2 agonist like loperamide, was tested in 2 RCTs using earlier diagnostic criteria. In one crossover trial, 2 doses (8 or 16 mg) showed no improvement in global symptoms or stool consistency vs placebo62. A 2nd Mexican trial (1997) compared 4 IBS groups (Manning criteria) with lidamidine or placebo, with or without psychotherapy, and reported significant improvement with lidamidine (89.5% vs 65.8%; p = 0.02), though no significant added benefit with psychotherapy63.
Several bile acid sequestrants (cholestyramine, colestipol, colesevelam, and obeticholic acid) have shown reduced colonic transit and improved stool consistency in BAD, but evidence in IBS-D is indirect, as they are mostly used as a therapeutic test13,24,25,64. Recently, liraglutide, a glucagon-like peptide-1 (GLP-1) analogue used in obesity and diabetes, showed similar benefit to colesevelam in a non-inferiority trial in BAD, though no studies exist in IBS-D65. Among emerging therapies targeting intestinal secretion, ibodutant—a selective neurokinin-2 receptor antagonist (family of neuropeptides mediating motility and nociception)—demonstrated dose-dependent benefit in women with IBS-D66.
Therapies targeting neuromodulation of the brain-gut axis
One of the key pathophysiological mechanisms in all IBS subtypes is neuroimmune dysfunction of the brain-gut axis. Neuromodulators are among the most effective tools across all IBS subtypes, modulating central and peripheral signaling, perception, and pain processing67–69. In general, neuromodulators are useful for pain control in any IBS subtype. In IBS-D, tricyclic antidepressants (TCAs) (imipramine, amitriptyline, desipramine, nortriptyline) are the most studied group, and have shown superiority over placebo for global improvement and pain scores, with NNT between 3 and 470. The recent ATLANTIS trial demonstrated that amitriptyline is effective for pain control across all IBS subtypes, and current evidence suggests it does not worsen constipation in IBS-C71. Due to its effects on muscarinic, adrenergic, and histamine receptors, amitriptyline reduces diarrhea frequency but may cause side effects such as dry mouth, drowsiness, and constipation. Therefore, gradual titration is recommended, aiming for maximum benefit between weeks 6 and 868,69.
Selective serotonin reuptake inhibitors (SSRIs) (sertraline, paroxetine, citalopram, escitalopram) are recommended in IBS for managing anxiety and hypervigilance symptoms, as their antinociceptive effect is limited, and they may decrease stool consistency69.
Serotonin-norepinephrine reuptake inhibitors (SNRIs) (duloxetine, venlafaxine, desvenlafaxine, milnacipran) are alternatives for pain control in IBS-D when TCAs are not well tolerated. An open-label study reported that 12 weeks of duloxetine improved abdominal pain scores, urgency, and bloating72.
Delta-ligands (pregabalin and gabapentin) are peripherally acting neuromodulators. Pregabalin has been evaluated in three RCTs in IBS, showing increased rectal sensory thresholds in hypersensitive patients, improved defecatory desire thresholds, and reduced pain scores73–75. One trial also reported decreased diarrhea frequency75. Evidence for other neuromodulators in IBS is scarce. Anecdotal case reports suggest mirtazapine (a tetracyclic antidepressant) may be useful in IBS-D, but there is no evidence for azapirones or atypical antipsychotics11,67,69.
Conclusions
IBS-D accounts for 9-40% of IBS cases. Its pathophysiology is multifactorial, including genetic predisposition, food intolerance, dysbiosis, low-grade inflammation, intestinal barrier and permeability alterations, neuroimmune dysfunction, and altered central sensory processing. As part of chronic diarrhea evaluation, medication-induced, infectious, inflammatory, and malabsorptive causes should be excluded through a basic targeted workup. Therapeutic options should address both pathophysiology and symptom control, and may include dietary restrictions, antidiarrheals acting on opioid receptors (loperamide and eluxadoline), local antibiotics such as rifaximin, probiotics, 5-HT3 antagonists (alosetron, ramosetron, ondansetron), bile acid sequestrants (cholestyramine), and neuromodulators, particularly TCAs. Evidence for mesalazine and lidamidine is weaker.
Funding
The authors declare that they received no funding for this study.
Conflicts of interest
O. Gómez-Escudero: speaker for Adium, Alfasigma, Carnot, Chinoin, Faes Farma. R. Carmona-Sánchez: participation in a research protocol with Laboratorios Senosiain.
Ethical considerations
Protection of humans and animals. The authors declare that no experiments on humans or animals were conducted for this research.
Confidentiality, informed consent, and ethics approval. This study does not involve personal patient data and does not require ethics approval. SAGER guidelines: Not applicable.
Declaration on the use of artificial intelligence: The authors declare that no generative AI tools were used in the drafting of this manuscript.
