Introduction
Irritable bowel syndrome (IBS) is a complex, multifactorial disorder in which various external factors—such as diet, GI infections, and environmental exposures—interact to trigger physiologic alterations in predisposed individuals. This susceptibility has been linked to intrinsic patient factors, including genetic predisposition, visceral hypersensitivity, altered pain perception, gut–brain axis dysfunction, food intolerances, and changes in gut microbiota composition (Figure 1). Because IBS is a heterogeneous condition, many of these factors may overlap. For example, low-grade inflammatory changes have been identified in the intestinal epithelium, possibly modulated by a specific microbiota, which itself can be affected by dietary changes. Consumption of foods that promote gas production and metabolites such as acetate and hydrogen sulfide may compromise epithelial barrier integrity, induce direct cellular damage, and trigger low-grade immunohistologic changes. This review focuses on traditional pathophysiologic mechanisms of IBS as well as those proposed in recent years.
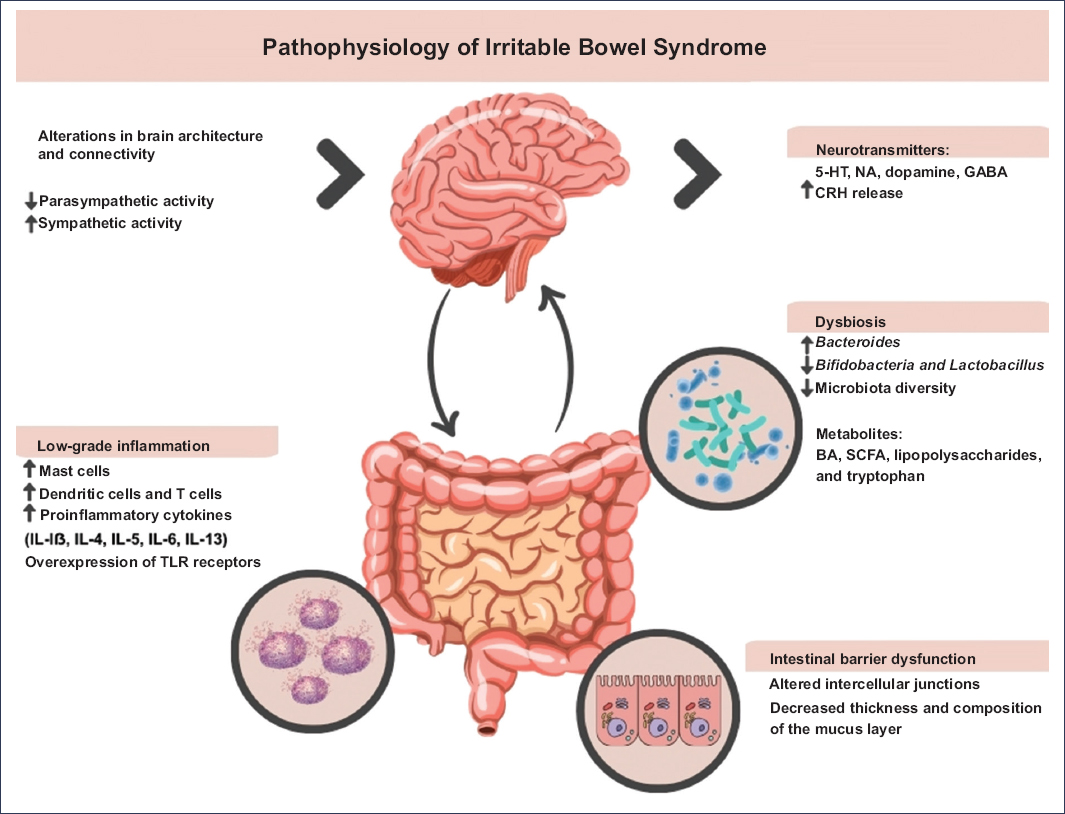
Figure 1. The pathophysiology of irritable bowel syndrome is multifactorial and involves a complex interplay of microbiota-gut-brain axis dysfunction, alterations in GI motility, visceral hypersensitivity, dysbiosis, low-grade immune dysfunction, and epigenetic changes. BA: bile acids; SCFAs: short-chain fatty acids; CRH: corticotropin-releasing hormone; GABA: gamma-aminobutyric acid; 5-HT: serotonin (5-hydroxytryptamine); IL: interleukin; NA: norepinephrine; TLR: Toll-like receptors.
Visceral hypersensitivity
Of all the described mechanisms, hypersensitivity is considered the most common and has been proposed as a hallmark finding in IBS, with approximately 60% of patients exhibiting increased intestinal sensitivity to physiologic stimuli1. Visceral hypersensitivity leads to exaggerated perception of distension, abdominal pain, and motility alterations, which are variable and fluctuate over time.
The exact cause remains unknown; however, several mechanisms involving peripheral and central sensitization have been proposed. These include dysregulation of neurotransmitters such as serotonin, which plays a critical role in intestinal motor coordination, as well as the influence of local mediators produced by immune cells and mast cells2.
Sensory nerve endings in the intestinal mucosa and submucosa detect nociceptive stimuli through ion channels (calcium, potassium, sodium), transient receptor potential (TRPV1, TRPA1, TRPV4) channels, proprioceptive receptors, and G protein–coupled receptors3. Sensitization of these channels is mainly modulated by mediators such as histamine and serotonin, which play key roles in amplifying pain signaling.
In addition, activation of protease-activated receptor 2 (PAR-2) by mast cell–derived proteases such as trypsin and tryptase promotes endocytosis processes and initiates signaling cascades that establish sustained nociceptive hypersensitivity4.
Intracellular signaling pathways mediated by p38 MAPK and protein kinase C are crucial for regulating ion channel and receptor expression in nerve terminals during inflammation. These pathways phosphorylate key proteins, thereby modifying the density and function of TRP and voltage-gated channels. Additionally, phosphorylation mobilizes channels from intracellular compartments to the membrane, increasing neuronal sensitivity to stimuli. Furthermore, activation of these pathways enhances expression of pronociceptive receptors, which detect inflammatory mediators and perpetuate excitability of the peripheral nervous system5.
The precise link between stress-induced sensitization and IBS remains unclear. Evidence suggests that repeated colonic distension induces increased motor activity and promotes secretion of corticotropin-releasing factor (CRF)6. Consequently, repetitive peripheral stimuli may activate stress hormones implicated in stress-related sensitization. CRF acts on extrahypothalamic sites within the central nervous system (CNS) that regulate behavior and autonomic responses. Anxiety and depression are also linked to CRF–hypothalamic axis dysregulation. For example, in rodents, CRF administration increases anxiety, stimulates motility, secretion, and colonic hypersensitivity7. In humans, CRF induces visceral hypersensitivity and increases colonic motility, whereas CRF antagonists alleviate these responses8.
Alterations in intestinal permeability
The integrity of the intestinal barrier is fundamental for protecting the organism against pathogens and preventing excessive inflammatory responses. In IBS, disruption of this barrier has been documented, particularly in the intercellular junctions between enterocytes. Electron microscopy studies have revealed separation of these junctions in biopsies from patients with IBS9. Similarly, histologic analyses of colonic biopsies have shown decreased expression of key tight junction proteins, including occludin, claudins 1, 3, and 5, and zonula occludens proteins9 (Figure 2).
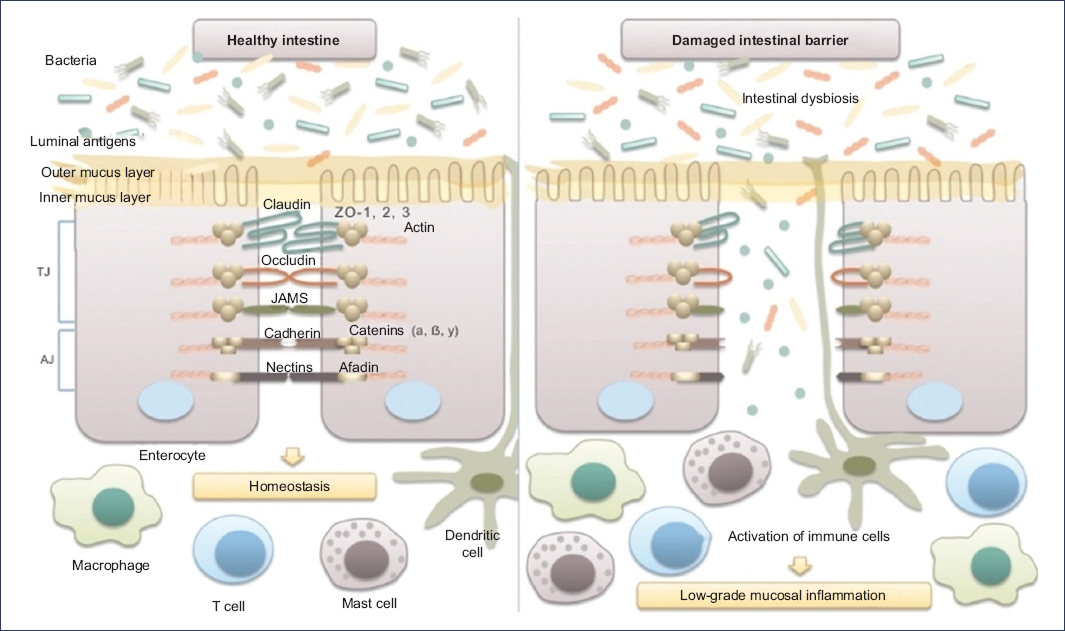
Figure 2. Microbiota and intestinal barrier integrity. The intestinal barrier plays an essential role in maintaining host homeostasis. It is composed mainly of the mucus layer, the epithelial layer, and the underlying lamina propria. Intestinal epithelial cells are tightly joined by junctional complexes. Tight junctions (TJ) are formed by several proteins, including occludin, claudins, zonula occludens (ZO), and junctional adhesion molecules (JAMs), which interact with one another and with the cellular cytoskeleton. Adherens junctions (AJ) are composed of the nectin–afadin system and the E-cadherin–catenin system. The integrity of the intestinal epithelial barrier prevents the translocation of luminal bacteria and antigens into the mucosa, thereby avoiding interaction with the host immune system and the development of low-grade mucosal inflammation in the intestinal wall (translated and adapted from Mamieva et al.9).
Conversely, expression of occludin and claudins 3 and 4 has been reported to increase in the presence of short-chain fatty acids (SCFAs), which are produced predominantly by microorganisms of the genera Eubacterium, Clostridium, Ruminococcus, and Faecalibacterium10.
Polyamines, particularly putrescine, spermidine, and spermine, are positively charged molecules produced by various bacteria. These polyamines have been shown to stimulate synthesis of E-cadherin and zonula occludens-1, 2 proteins essential for maintaining barrier integrity. E-cadherin promotes adhesion between epithelial cells, whereas zonula occludens-1 contributes to tight junction formation, preventing the passage of intraluminal substances into the host3,11. Certain intestinal bacteria also influence the thickness and composition of the intestinal mucus layer, including Bacteroides thetaiotaomicron, Faecalibacterium prausnitzii, and species of the genus Ruminococcus.
Microbiota-related alterations and changes in the synthesis of proteins involved in the intestinal barrier contribute to increased intestinal permeability, allowing translocation of luminal antigens, microbial components, and metabolites, which continuously stimulate the immune system. As a result, T lymphocytes and mast cells in the intestinal mucosa are activated, and inflammatory mediators proliferate. Notably, elevated levels of proinflammatory cytokines, such as interleukins (IL)-6 and IL-8, along with attenuated responses of anti-inflammatory cytokines, such as IL-10, have been reported.
Comparative clinical trials with healthy controls have demonstrated that patients with IBS show higher expression of TGR5 (Takeda G protein-coupled receptor) and significantly high concentrations of SCFAs and bile acids in fecal samples, findings linked to altered absorption and intestinal motility. Elevated tryptophan levels have also been identified, suggesting activation of the aryl hydrocarbon receptor pathway, potentially contributing to altered signaling and visceral sensitivity.
Dysbiosis and irritable bowel syndrome
The intestinal microbiota is essential for maintaining host homeostasis. About 90% of the bacterial population in adults is predominantly composed of Firmicutes, Bacteroides, actinobacteria, and proteobacteria—microorganisms involved in key metabolic functions such as the metabolism of carbohydrates, amino acids, and lipids, as well as the synthesis of cholesterol and vitamins (K2, B1, B2, B6, B7, B9, and B12). In addition, the microbiota maintains the integrity of the epithelial barrier and plays a role in modulating the immune response and protecting against pathogens9,12.
Numerous studies have shown that patients with IBS have an increased abundance of bacterial genera such as Bacteroides, while populations considered beneficial, such as Bifidobacteria, Lactobacillus, and Clostridium, are significantly reduced vs healthy individuals13. Although this dysbiosis pattern is not identical across all patients, most authors agree that there is a reduction in microbial diversity in IBS. Specifically, a lower abundance of butyrate-producing bacteria, such as those from the genus Faecalibacterium—particularly F. prausnitzii—has been described, along with an increase in the family Enterobacteriaceae, including pathogens such as Escherichia coli and species from the genus Enterobacter9. Significant differences have also been reported in Lactobacillus abundance; however, findings are inconsistent, as some studies report an increase while others show a decrease of this commensal11.
Dysbiosis in IBS patients has been shown to affect the production of metabolites such as bile acids, SCFAs, amino acid metabolites, and tryptophan derivatives14.
SCFAs, such as acetate, propionate, and butyrate, are produced through bacterial fermentation of carbohydrates. These metabolites are essential for enterocyte nutrition and play a key role in modulating the inflammatory response and maintaining the integrity of the intestinal barrier14. On the other hand, protein catabolism and subsequent absorption of amino acids—particularly tryptophan—constitute a relevant pathway in IBS pathophysiology. Through microbial hydrolysis and transformation, tryptophan acts as a precursor of serotonin via enzymes such as tryptophan hydroxylase. Moreover, indole metabolites derived from tryptophan can activate the aryl hydrocarbon receptor, thereby modulating the inflammatory response and regulating intestinal barrier integrity and permeability. Variations in the intestinal microbiota’s ability to metabolize tryptophan may influence serotonin production, directly affecting visceral sensitivity and intestinal motor function, as described above14.
Bile acids act as signal integrators between cholesterol metabolism, liver function, and microbial activity. They are synthesized in the liver as primary bile acids (cholic acid and chenodeoxycholic acid) via enzymatic activity of cholesterol 7α-hydroxylase (CYP7A1) and are further modified in the intestinal lumen into secondary bile acids (such as deoxycholic acid) by specific bacteria, particularly of the genus Clostridium. These metabolites modulate motility and sensitivity through receptors such as the farnesoid X receptor and the G protein–coupled receptor. Changes to these processes—for example, fatty acid malabsorption in diarrhea-predominant IBS (IBS-D)—have been correlated with increased serotonin production and colonic motility dysfunction14. Some studies have shown that IBS-D patients have elevated levels of primary bile acids and reduced levels of secondary bile acids, a phenomenon associated with reduced Ruminococcaceae family abundance and with symptoms of diarrhea and visceral hypersensitivity15.
The role of infectious agents
In the past decade, evidence has emerged suggesting that infections may play a key role in the pathophysiology of IBS. The most important aspects of this evidence are summarized below.
Antibiotic use and IBS
Inappropriate antibiotic prescribing, to which the general population is frequently exposed, has been associated with the development of GI symptoms. Antibiotics are the most common and significant cause of alterations in the intestinal microbiota16. The potential of an antimicrobial agent to affect the intestinal microbiota depends on several factors, including its spectrum of activity, dose, pharmacokinetics, and duration of administration. Importantly, the parenteral route is not exempt from altering the microbiota, since many of these drugs (eg, ceftriaxone) can be excreted in bile and saliva.
Regarding evidence linking antibiotic overuse with digestive symptoms, one study surveyed 421 subjects in a primary care practice in the United Kingdom who had received antibiotics within a 4-month period17. This study demonstrated that antibiotic use was strongly associated with up to a 3-fold increased risk of developing IBS symptoms compared with subjects who had not taken antibiotics (OR, 3.7).
Postinfectious IBS
Although this concept may be relatively new in terms of scientific rigor, more than 40 years ago McKendrick and Read18 first reported the late onset of GI symptoms following episodes of bacteriologically confirmed acute gastroenteritis. Since then, numerous studies have confirmed this relationship19. Recently, the Rome Foundation convened a working group that conducted a systematic review of the literature addressing the clinical features, pathophysiology (sensorimotor alterations, dysbiosis, immune dysfunction, epithelial barrier changes, enteroendocrine pathways, and genetics), and animal models of postinfectious IBS (PI-IBS), and proposed specific criteria for its definition20,21 (Table 1).
Table 1. Diagnostic criteria for post-infectious irritable bowel syndrome
| For the diagnosis of post-infectious irritable bowel syndrome, the following Rome IV-based criteria are considered: |
|---|
| – Recurrent abdominal pain at least 1 day per week in the last 3 months, with symptom onset at least 6 months before diagnosis, associated with two or more of the following: |
| • Pain related to defecation |
| • Association with a change in stool frequency |
| • Association with a change in stool form (appearance) |
| – Development of symptoms immediately after resolution of an infectious gastroenteritis. |
| – Definition of infectious gastroenteritis: |
| • Confirmation by a positive stool culture in a symptomatic individual, or |
| • Presence of at least two of the following symptoms when culture is not available: |
| ⦿ Fever |
| ⦿ Vomiting |
| ⦿ Diarrhea |
| – Absence of a prior diagnosis of irritable bowel syndrome before the onset of the acute illness. |
A systematic review and meta-analysis including 47 studies with more than 28,000 individuals reported a global prevalence of 14.5% for PI-IBS and 12.7% for postinfectious functional dyspepsia22. The risk of developing PI-IBS or postinfectious functional dyspepsia was significantly higher among those with prior gastroenteritis (OR, 4.3 and 3.0, respectively) compared with unexposed controls. PI-IBS persisted in nearly 40% of cases even after 5 years. Parasitic infections showed the highest prevalence of PI-IBS (30.1%), followed by bacterial (18.3%) and viral (10.7%) infections. Campylobacter was the bacterial pathogen most strongly associated (20.7%), while SARS-CoV-2 and certain Proteobacteria were linked to the greatest risks (OR, 5.4). These findings reinforce the role of proinflammatory infectious agents as key triggers of persistent functional GI alterations.
The mechanism associated with the development of IBS after infection seems to involve perpetuation of a low-grade inflammatory response throughout time23. This response is characterized by cytokine release, increased intraepithelial lymphocytes, and infiltration of mast cells and eosinophils into the jejunal and colonic mucosa, including deeper layers where nerve endings are located. For example, Chadwick et al24 evaluated colonic mucosa samples from 77 patients with IBS and found microscopic inflammation in 31, with immunohistology revealing increased intraepithelial lymphocytes as well as higher numbers of CD3+ and CD25+ cells in the lamina propria, which is consistent with immune activation. These inflammatory changes may result from exposure to bacterial antigens or dietary components25,26. On the other hand, there may be a predisposition to an exaggerated intraluminal inflammatory response, based on studies demonstrating the presence of gene polymorphisms that encode for the production of anti-inflammatory cytokines.
Furthermore, Pimentel et al27 described that cytotoxin distending toxin B (CdtB), produced by pathogens such as Campylobacter, Shigella, and Escherichia coli, can trigger a cross-reactive immune response generating antibodies against vinculin, a key linker protein for smooth muscle contractility. In this model, infections are proposed as a cause of dysmotility in certain cases of IBS. Currently, testing for antivinculin and anti-CdtB antibodies is recommended in patients with IBS-D or IBS-M, as validation studies demonstrated that values above the cutoff can differentiate IBS from other conditions, such as inflammatory bowel disease.
Interestingly, since the COVID-19 pandemic, it has been documented that up to 7% of infected individuals develop IBS (95% CI, 5-8), with the highest likelihood within the first 3 months (6%; 95% CI, 2-10) and at 6 months (7%; 95% CI, 5-8), predominantly IBS-D (5%; 95% CI, 1-8)18.
The genesis of IBS in post-COVID-19 patients seems to result from a complex interaction of multiple mechanisms. SARS-CoV-2 initially enters GI epithelial cells via angiotensin-converting enzyme 2 (ACE2) receptors, highly expressed in the small intestine, particularly the terminal ileum and duodenum. This invasion promotes local viral replication and direct epithelial injury, compromising barrier integrity.
Disruption of the intestinal barrier favors altered ion transport and nutrient absorption, along with induction of an inflammatory response characterized by increased cytokines, such as IL-8, leading to enhanced intestinal permeability and abnormal immune activation. These changes are associated with the visceral hypersensitivity typical of IBS. Additionally, SARS-CoV-2 infection is linked to intestinal dysbiosis, evidenced by reduced microbial diversity and richness, increased opportunistic pathogens, and decreased beneficial bacteria. Loss of ACE2 regulatory function in amino acid absorption—such as reduced tryptophan uptake—further limits antimicrobial peptide synthesis, exacerbating microbial imbalance and local inflammation19.
Moreover, acute and chronic stress during infection and confinement, as well as disease-related uncertainty, activate the hypothalamic-pituitary-adrenal axis, resulting in corticotropin release and increased catecholamines. This process alters intestinal motility, modifies neurotransmitter secretion (eg, serotonin), and impairs mucosal barrier function.
Finally, treatments used for COVID-19—including broad-spectrum antibiotics (azithromycin, vancomycin, ceftriaxone), antivirals (remdesivir, lopinavir/ritonavir), hydroxychloroquine, corticosteroids, and frequent polypharmacy—have been associated with persistent dysbiosis and alterations in barrier function and permeability, facilitating luminal antigen translocation and activation of the local immune system19.
IBS and bacterial overgrowth
This topic will be addressed in detail in another article in this series. Nevertheless, it is important to highlight that the role of bacteria in IBS has been supported by the hypothesis that up to 80% of patients with IBS present with bacterial overgrowth28–30. This paradigm suggests that bacterial overgrowth may be associated with abnormalities in small bowel motor function and may underlie symptoms—particularly abdominal bloating and distension. Moreover, eradication of this bacterial overgrowth with nonabsorbable antibiotics (eg, neomycin, rifaximin) has been associated with symptomatic improvement in more than 80% of patients31.
Low-grade inflammation and the immune system
From an immunologic perspective, IBS is characterized by subtle but significant activation of effector cells, particularly near nerve fibers, where they release mediators such as histamine, tryptase, and proteases. These mediators not only alter intestinal contractility but also contribute to the sensitization of nerve endings and the manifestation of abdominal pain2. Increased numbers and activation of mast cells have been identified, a phenomenon that can occur through immunoglobulin E (IgE)–dependent or–independent pathways, underscoring the complexity of the inflammatory response.
The interaction between mast cells and nerve terminals gives rise to neurogenic inflammation, mediated by the release of neuropeptides such as substance P and calcitonin gene-related peptide. These neuropeptides induce mast cell degranulation, amplify the release of inflammatory mediators, and further sensitize nociceptive neurons3.
Cytokines also play an important role here. Proinflammatory cytokines, such as IL-1β and IL-6, coexist with anti-inflammatory or tolerogenic cytokines, such as IL-10 and transforming growth factor β, which are normally produced by epithelial, stromal, and immune cells to maintain intestinal homeostasis. Other cytokines, such as IL-4, IL-5, and IL-13, promote local allergic responses and mast cell activation32.
A key mechanism in the intestinal immune response is the activation of Toll-like receptors (TLRs), which recognize microbial components known as pathogen-associated molecular patterns. These receptors, expressed on both epithelial and immune cells in the mucosa, trigger an intracellular cascade upon stimulation, culminating in nuclear factor κB activation and release of cytokines and inflammatory mediators. Under normal conditions, this process contributes to immune tolerance toward commensal microbiota while preserving responsiveness to pathogens. However, in IBS, overexpression of TLR-4 and TLR-5—receptors that detect bacterial lipopolysaccharides and flagellins, respectively—has been observed. This overexpression facilitates exaggerated immune responses even to subtle microbiota alterations, promoting production of cytokines such as IL-6 and tumor necrosis factor α33.
Histamine, synthesized from histidine via histidine decarboxylase, plays a central role in immune and inflammatory responses. In the intestine, histamine is released mainly by mast cells and basophils, although some microorganisms expressing this enzyme can also produce it. Of particular interest in IBS are histamine receptors H1 and H4 which are involved in the sensitization of nerve endings, partly by activating channels such as TRPV1, thereby contributing to pain transmission34.
Altered intestinal motility
Disorders of GI motility are a fundamental component of IBS pathophysiology. Multiple studies have documented abnormalities in both small bowel and colonic motor activity. In the small bowel, patterns of either accelerated or delayed transit have been observed, contributing to manifestations such as diarrhea or abdominal bloating35. In the colon, patients with IBS exhibit alterations in the frequency, amplitude, and propagation of motor contractions, particularly propulsive contractions, leading to changes in transit and bowel evacuation. These motor dysfunctions seem to be modulated in part by abnormal regulation of the enteric nervous system and the gut–brain axis, as well as by luminal factors (eg, microbiota) and hormonal influences (eg, intestinal peptides)36. In IBS-D, colonic hypermotility and accelerated transit predominate, whereas in IBS-C, reduced motility and delayed transit are observed1. Specifically, evidence indicates an exaggerated colonic motor response to stimuli such as food intake or rectal distension, a phenomenon referred to as postprandial motor hypersensitivity37. Similarly, impaired anorectal coordination has been identified, contributing to sensations of incomplete evacuation or defecatory difficulty, particularly in IBS-C patients38. Overall, these motor alterations reflect the interplay of neuromuscular dysfunction, visceral hypersensitivity, and low-grade immune and inflammatory influences that characterize IBS.
Gut–brain axis: neurotransmitters and stress
One of the fundamental pillars in the pathophysiology of IBS is the alteration of bidirectional communication between the central nervous system (CNS) and the GI tract (Fig. 3). Under normal conditions, this axis allows coordinated regulation of motor, sensory, and secretory functions of the digestive system. In patients with IBS, various neuroimaging techniques have revealed abnormalities in the architecture and connectivity of several brain networks, including:
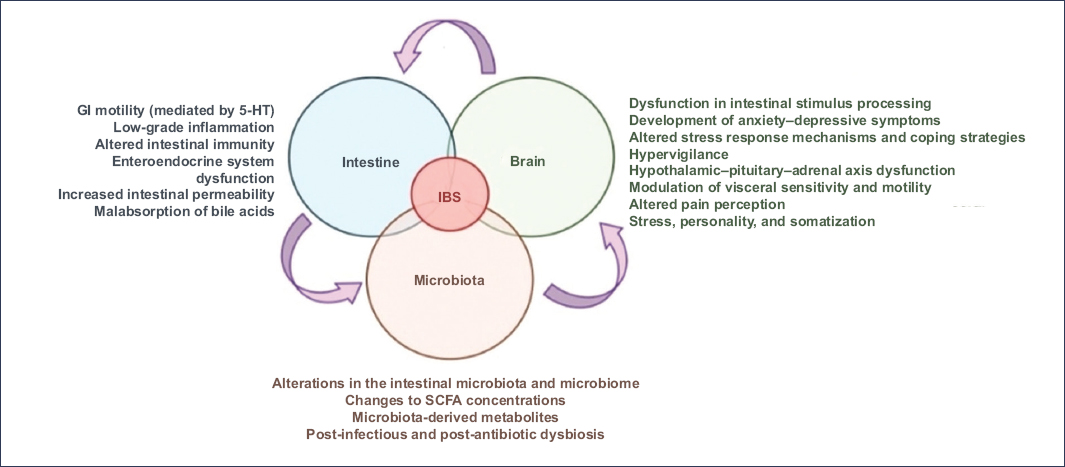
Figure 3. The microbiota-gut-brain axis refers to the bidirectional communication pathways between the central nervous system, the enteric nervous system, the GI tract, and the intestinal microbiota. When disrupted, this complex interaction network contributes to various pathophysiological mechanisms underlying irritable bowel syndrome.
SCFAs: short-chain fatty acids; 5-HT: serotonin (5-hydroxytryptamine) (translated and adapted from Pellegrino et al.12).
- – Default mode network: comprising the medial prefrontal cortex, posterior cingulate cortex, precuneus, inferior parietal cortex, lateral temporal cortex, and hippocampal formation. This network is responsible for self-reflection, episodic memory processing, and internal thought management. In IBS, dysfunction of this network—evidenced by altered structural and functional connectivity—may contribute to impaired modulation of visceral pain and abnormal attribution of attention to internal stimuli, which is associated with symptom intensity and chronicity24.
- – Salience network: responsible for detecting, filtering, and prioritizing relevant stimuli from both the internal environment (visceral sensations) and external environment. It primarily involves the medial and orbitofrontal prefrontal cortex, mid-anterior cingulate cortex, anterior insula, and amygdala. In IBS, hyperactivation of this network has been observed, for example during rectal distension, a phenomenon associated with greater pain perception and enhanced emotional processing of visceral signals24,25.
- – Central executive network: engaged in higher-order cognitive functions such as attention, working memory, and inhibitory control. It is associated with the lateral prefrontal cortex and posterior parietal areas. In IBS, deficits in connectivity and activation of this network during cognitive tasks or in response to painful stimuli have been reported, potentially leading to disproportionate attention to symptoms and impaired modulation of pain responses24,26.
- – Central autonomic network: regulates body homeostasis through the control of visceromotor responses, neuroendocrine signaling, and pain processing. It includes structures such as the insular cortex, amygdala, hypothalamus, periaqueductal gray, and locus coeruleus. In IBS, dysfunction translates into altered autonomic modulation.
- – Emotional processing networks: responsible for emotional evaluation and responses, including the amygdala, hippocampus, hypothalamus, and prefrontal cortex. Their dysfunction in IBS is associated with greater anxiety, exaggerated pain perception, and increased reactivity to interoceptive stimuli24.
Finally, along the brain-gut axis, neurotransmitters such as serotonin, norepinephrine, and dopamine play a crucial role not only in the pathophysiology but also as targets of current therapeutic strategies
Several studies have demonstrated that chronic stress and emotional disorders, such as anxiety and depression, are associated with alterations of the intestinal microbiome, which can in turn exacerbate GI symptoms, and vice versa39.
Adverse childhood experiences—including emotional abuse (particularly in women), sexual abuse (particularly in men), and substance abuse within the family environment—are linked to increased risk of developing IBS. This association is mediated by hyperactivity of the hypothalamic-pituitary-adrenal axis, leading to dysfunction of the gut-brain axis. In a study by Lee et al,40 the presence of at least one adverse childhood experience doubled the risk of IBS in both women and men (overall OR, 2.11; 95% CI, 1.58-2.82; p = 5.09E-7). Each unit increase in the childhood adverse experiences score increased risk by 18%. Among specific categories, household mental illness showed the strongest statistical association, significantly increasing risk in both women (OR, 1.95; 95% CI, 1.35-2.85; false discovery rate [FDR], 0.002) and men (OR, 2.32; 95% CI, 1.26-4.33; FDR, 0.014). Emotional abuse was a particularly strong predictor in women (OR, 1.94; 95% CI, 1.23-3.09; FDR, 0.019), whereas sexual abuse was a determinant in men (OR, 3.54; 95% CI, 1.35-10.38; FDR, 0.027)40.
In a cross-sectional study conducted in Mexico including 290 adults (90 with IBS and 200 healthy controls), the prevalence of childhood adverse experiences and their association with IBS were evaluated41. Participants completed validated questionnaires for adverse experiences, visceral sensitivity, IBS severity, and anxiety and depression symptoms. Results showed that 80% of IBS patients reported at least one adverse experience, vs 59% of controls (p < 0.0001). Moreover, 75% of IBS patients with severe symptoms reported ≥ 4 adverse experiences, which was associated with higher risk of IBS. Individuals with adverse experiences presented higher levels of anxiety and depression. These findings underscore the high prevalence of childhood adverse experiences among Mexicans with IBS and the importance of incorporating their evaluation into the comprehensive management of this condition in Latin American populations.
Genetics and epigenetics in irritable bowel syndrome
Regarding genetic factors, gene variants have been identified that are associated with susceptibility to IBS, as well as with neurotransmitters, inflammatory processes, and intestinal motility. Having a family member with IBS has been strongly associated with the development of IBS in adults (OR, 2.17; 95% CI, 1.89-2.49; p < 0.0001)42. Twin studies have also recognized the heritability of IBS, with higher concordance in monozygotic twins (33%) than in dizygotic twins (13%)43.
In the study by Bonfiglio et al44, the locus 9q31.2 (single nucleotide polymorphism rs10512344) was documented to be associated with the risk of IBS, particularly in relation to the IBS-C subtype in women. This locus has been primarily linked to the regulation of cellular ion transport membranes, mutations in the sucrase-isomaltase gene, and autonomic dysfunction12.
In another study, Huang et al45 identified 10 risk loci for IBS, of which 7 were novel. Some encoded the following genes: COP1, LRP1B, SUGT1, MED12L, P2RY14, and SHISA6. They also confirmed previously known IBS-related genes, including PRRC2A, CADM2, and PHF2. In particular, PRRC2A maps to the variant rs2736155 and has been linked to intestinal immune response, potentially influencing both susceptibility and pathological response in IBS45.
In addition, epigenetic mechanisms have been identified that play a fundamental role in synaptic plasticity, pain mechanisms, and depression. Epigenetic changes involved in this disorder include DNA methylation, histone modification, and noncoding RNA-mediated gene regulation46.
DNA methylation involves the addition of a methyl group (–CH3) to the C5 position of cytosine, particularly in cytosine-phosphate-guanine (CpG) dimers. Regions with high CpG content, often located in gene promoters, are typically unmethylated. Methylation, therefore, contributes to chromatin closure and repression of gene transcription. In patients with IBS, altered methylation patterns have been detected in genes related to stress response (such as the glucocorticoid receptor) and other genes involved in neuronal function46.
Histones, which are essential proteins in chromatin organization that enable DNA winding, can undergo diverse covalent changes, including acetylation and methylation. These epigenetic changes are sensitive to environmental factors such as stress, which can alter these processes and, depending on the affected gene regions, influence modulation of visceral pain46.
Regarding noncoding RNA, 2 types are most relevant: microRNAs (miRNAs) and long noncoding RNAs (lncRNAs). miRNAs are small noncoding RNA molecules, approximately 18-25 nucleotides in length, that regulate post-transcriptional gene expression. Their main mechanism is binding to the 3′-untranslated region (3′-UTR) of target mRNAs, leading to degradation or inhibition of translation. Several miRNAs, including miR-510, miR-29a, and miR-144, have shown altered levels in both IBS patients and experimental models47. These miRNAs regulate key genes involved in intestinal permeability, visceral pain sensitivity, and serotonin neurotransmitter function.
lncRNAs are transcripts longer than 200 nucleotides that, although not coding for proteins, play key roles in regulating transcriptional and translational processes. These RNAs can interact with regulatory proteins and participate in chromatin remodeling, thereby modulating gene expression. Relevant lncRNAs, such as XIST and GHRLOS, have been identified and involved in the regulation of serotonin transporter and motilin expression, contributing to understanding the mechanisms of visceral hypersensitivity and GI motility alterations associated with IBS46.
Novel pathophysiological mechanisms
Beyond the classical mechanisms—such as alteration of the gut–brain axis, dysbiosis, and visceral hypersensitivity—emerging elements have been identified that may play a relevant role in the development and perpetuation of the disease.
Among these, the impact of consuming ultra-processed foods stands out, as their increasing incorporation into the Western diet has been correlated with a rise in the incidence of IBS. Likewise, environmental exposure to atmospheric pollutants and hygiene conditions during childhood have been linked to immune and inflammatory alterations that favor intestinal dysfunction.
In a particularly novel approach, it has been proposed that gravity—as a constant physical force that affects the distribution of organs, blood circulation, and pressure gradients in the gastrointestinal tract—may also contribute to the development of functional symptoms by altering both intestinal motility and visceral sensitivity in susceptible individuals.
The integration of these new concepts allows for a broader and more complex view of IBS, highlighting the interaction of dietary, environmental, immunological, microbiological, and physical factors in its pathophysiology, and opening the door to new strategies for prevention and treatment.
Processed food consumption
The increase in IBS prevalence has coincided with the rise in consumption of ultra-processed foods. In a study by Wu et al48, a higher intake of ultra-processed foods was associated with greater risk of developing IBS. Specifically, each 10% increase in the proportion of ultra-processed foods in the diet was associated with an 8% increased risk of IBS (HR, 1.08; 95% CI, 1.04-1.12).
Several mechanisms may explain the association between ultra-processed food consumption and IBS. These foods often contain high levels of sugars, saturated fats, and calories, along with low levels of fiber, vitamins, and micronutrients. Such composition can disrupt energy regulation and promote a proinflammatory state in the intestinal mucosa. Ultra-processed foods can contain high concentrations of oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs). Excess FODMAP intake increases water volume in the small intestine and gas production in the colon, generating IBS-like symptoms—a topic discussed in detail in another article in this series. One of the most important mechanisms involves food additives (emulsifiers, thickeners, preservatives), which can alter the composition and function of the intestinal microbiota, reducing short-chain fatty acid (SCFA) production, crucial for maintaining mucosal integrity48.
Environmental exposure
Recent studies have reported an association between air pollution (particularly fine particulate matter [PM 2.5] and toxic emissions) and increased rate of IBS. Inhaled microparticles can cross the alveolar barrier, enter the bloodstream, and reach the digestive system. Exposure to these particles may trigger systemic inflammatory responses and increase oxidative stress, damaging the intestinal mucosa and impairing local immune function. Animal models have further shown that exposure to high concentrations of microparticles induces intestinal dysbiosis and disruption of the gut–brain axis49.
Other environmental factors include exposure to pets and childhood hygiene conditions. Observational studies have shown that pet ownership (particularly herbivores such as horses or birds) and inadequate hygiene during childhood predispose individuals to an immune profile skewed toward a Th2 response. This polarization may contribute to increased density of IgE+ mast cells and greater intestinal permeability, both linked to the development of an “atopic” IBS phenotype in which GI symptoms coexist with allergic signs49.
Gravitational hypothesis
An innovative approach recently proposed in IBS pathophysiology is the “gravitational hypothesis.”50 This concept integrates previously described theories—such as motility disturbances, gut–brain axis dysfunction, dysbiosis, and autonomic nervous system disorders—into a unifying model based on human interaction with gravitational force. According to this hypothesis, IBS may arise from failure of anatomical, physiological, and neuropsychological systems to adequately manage gravitational stress. From an anatomical perspective, structures such as the mesentery, taeniae coli, spine, rib cage, diaphragm, and anterior abdominal wall work together to suspend and stabilize abdominal organs against gravitational pull. When these mechanical support systems are impaired—as in hypermobility disorders, aging, or musculoskeletal alterations—processes such as dysmotility, luminal stasis, and microbial overgrowth may occur, all of which are relevant to IBS development.
In parallel, the hypothesis suggests that the perception of “gravitational stress” by the peripheral and central nervous systems—through visceral sensitivity and hypervigilance to internal stimuli—can amplify symptom response via peripheral and central sensitization. Hyperactivity of systems such as the cardiovascular baroreflex, the vestibular apparatus, or emotional processing networks may contribute to visceral hypersensitivity, abdominal pain, and heightened emotional responses typical of IBS. Serotonin, a key neurotransmitter in GI motility and pain modulation, is also central to this theory, acting as an evolutionary mediator in gravitational adaptation.
Overall, the gravitational hypothesis offers an integrative perspective linking mechanical, microbial, immune, and neuropsychological factors, proposing that impaired adaptation to the constant force of gravity may be a primary contributor to IBS pathogenesis. This perspective not only expands understanding of the disease but also opens new avenues for diagnostic and therapeutic research, focusing on improving biomechanics, modulating sensitivity to gravitational stress, and restoring intestinal homeostasis.
Conclusions
Since its original description, IBS diagnosis has been symptom-based, and accordingly, treatment has focused on symptom relief. From the Manning criteria to the Rome IV criteria, abdominal pain has remained the primary symptom of IBS; thus, therapy has traditionally targeted pain relief. However, it is now clear that pain reduction alone is insufficient, and relief of abdominal bloating and improvement in stool frequency and consistency must also be considered therapeutic goals in IBS. Moreover, IBS significantly affects quality of life and carries economic implications, both of which should be evaluated when assessing the therapeutic efficacy of pharmacological and non-pharmacological interventions.
Current IBS management is therefore considered integrative, aiming for overall symptom improvement, enhanced quality of life, and a favorable safety profile. Treatment may be tailored to the most bothersome symptom (abdominal pain or bloating), predominant bowel habit (diarrhea or constipation), or underlying pathophysiology (eg, psychiatric comorbidities, visceral hypersensitivity).
IBS must be understood as a complex and dynamic disorder, where classical mechanisms such as visceral hypersensitivity and dysbiosis coexist with emerging findings including epigenetic changes, prior infections, environmental factors, and dysregulation of the gut–brain axis. Integration of these mechanisms underscores the need for a comprehensive, personalized diagnostic and therapeutic approach, based on each patient’s clinical and pathophysiological profile.
Funding
The authors declared having received no financial support for this study.
Conflicts of interest
S.P. Mendivil-Saenz: no conflicts of interest. J.M. Remes-Troche: advisor and member of advisory boards for Asofarma, Carnot, Pro.Med.CS. Praha a.s., and Pisa; lecturer for Asofarma, Abbott, Carnot, Chinoin, Ferrer, Johnson & Johnson, Medix, and Medtronic.
Ethical considerations
Human and animal protection: The authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality, informed consent, and ethics approval: The study does not involve personal patient data and did not require ethics approval. SAGER guidelines do not apply.
Declaration on the use of artificial intelligence: The authors declare that no generative artificial intelligence was used in the preparation of this manuscript.
