Definition and physiological foundations
Irritable bowel syndrome (IBS) is a disorder of the gut-brain interaction (DGBI). The gut-brain axis is the complex bidirectional connection between the intestine and the central nervous system (CNS), which includes the brain, spinal cord, autonomic nervous system (sympathetic, parasympathetic, and enteric), neuroendocrine system, and neurohumoral system1. Afferent pathways transmit visceral sensitivity to the brain through 3 orders of neurons: the first from the intestine to the dorsal horn of the spinal cord, then from the spinal cord to the thalamus, and from there to the midbrain. Brain nuclei that regulate visceral pain include the nucleus of the solitary tract, parabrachial nucleus, locus coeruleus, rostral ventromedial medulla, anterior cingulate cortex, paraventricular nuclei, and the amygdala2. The brain can modulate sensations in a descending manner, modifying the sensitivity of the dorsal horns. A crucial component of the gut-brain axis is the autonomic nervous system. An increase or decrease in vagal activity, or in sympathetic activity, or the balance between both, can affect pain perception. Some factors, such as sweating, arrhythmias, and alterations in the respiratory cycle, as well as autonomic regulation disorders such as orthostatic hypotension syndrome, are frequent in patients with DGBI3.
Sensitivity
In healthy individuals, the function of the small intestine is not perceived. More than 90% of the afferent sensory information from the digestive tract has homeostatic functions and is not consciously perceived. The digestive tract is densely innervated and regulates digestion, absorption, and the detection of potential dangers4. Communication between the digestive tract and the CNS is both ascending and descending. Afferent pathways (from the gut to the brain) transmit information from visceral receptors to the brain, where the information is received and integrated, producing cognitive, emotional, and behavioral responses5. From the upper digestive tract, sensory afferents transmit signals of temperature, taste, hunger, satiety, nausea, and pain. In the lower digestive tract (intestine and colon), intestinal distension produces pain mediated by stretch receptors in the muscular layers and serosa, which project via the splanchnic and vagal nerves to the brain. Contractions produce nausea, bloating, cramping, discomfort, and pain5,6. Sensory neurons of the enteric nervous system activate local responses, while extrinsic afferent nerves transmit sensory information to the spinal cord or medulla for processing and integration, generally conducted via the vagus nerve and spinal afferents. The cell bodies of vagal afferents are located in the nodose ganglion (base of the skull) and project to the nucleus of the solitary tract. Vasovagal reflexes stimulate vagal efferents in the dorsal motor nucleus of the vagus, located in the medulla oblongata. The cell bodies of spinal afferents are located in the dorsal root ganglia and are divided into thoracolumbar and lumbosacral fibers; they synapse in the spinal cord and send information to the brainstem5.
Visceral pain processing is very different from somatic pain processing, which explains why visceral sensations are more diffuse yet very unpleasant, and can trigger psychological experiences of aversion. They are also highly modifiable by cognitive and emotional factors7. The cerebral processing of sensory information from the viscera involves the activation of multiple cortical and subcortical regions, known as central mediation or central processing8. This has been extensively investigated in IBS through imaging studies that, compared with healthy controls, show differences in the processing of painful sensations from the esophagus, stomach, and rectum. Patients with DGBI exhibit altered visceral perception, termed visceral hypersensitivity, in response to symptoms9. These abnormal responses involve multiple brain networks, including the sensorimotor, emotional, and salience networks8. The brain networks involved are modulated by stress-sensitive central pathways, such as the corticotropin-releasing factor pathways, the locus coeruleus, and noradrenergic pathways. Although the exact mechanisms by which abdominal pain develops in IBS patients remain unknown, several theories exist regarding predisposing factors (e.g., genetics, environmental factors, history of abuse, inflammation, and drugs) and perpetuating factors (persistent inflammation, microbiota changes, and psychological factors). In addition to episodic pain, patients with persistent pain may develop changes in the CNS that perpetuate it. The theoretical concept suggests that continuous pain transmission can modify CNS physiology and structure10. For this reason, in many patients, drugs targeting only the digestive tract—modifying motility or peripheral sensitivity—fail in treating IBS pain, and a second neuromodulator targeting the CNS or descending pathways may reduce it11. Patients with DGBI develop fear of symptoms due to their unpleasant and unpredictable nature, as well as aversion to situations associated with pain generation. These patients are characterized by hypervigilance, excessive fear of symptoms, and intense avoidance behaviors12. They fear situations related to the digestive tract, such as not having a bathroom nearby, which produces disability. Anxiety associated with GI symptoms can increase symptom severity and impair quality of life13. Several studies have demonstrated significant positive effects in IBS treatment when therapy targets the anxiety associated with GI symptoms14.
Effect of food
Nutrients in the digestive tract promote GI motility, secretion, absorption, and sensitivity. Fat is a very active component, with strong effects on both sensitivity and motility5. Patients with DGBI are more sensitive to fat in the small intestine than healthy controls, since an isocaloric meal of another type does not produce the same response15. Response to food also has cognitive and emotional components, as it provides satiety, well-being, and mood improvement. However, patients with DGBI exhibit abnormal function, with increased sensitivity and symptom perception in response to physiological stimuli16. In one study, when patients were misinformed about the fat content of a test meal, their symptoms worsened despite being given a low-fat yogurt17.
Genetics
Genetic mechanisms are supported by family aggregation and twin studies18.
Somatization
Somatization is the tendency to express psychological distress as somatic (physical) discomfort rather than as emotional distress19. Emotional blocking is a common component in patients who somatize and is considered a defense mechanism to avoid anxiety associated with intense or conflicting feelings20. Alexithymia refers to a generalized difficulty in consciously understanding one’s own emotions. It is common in individuals with IBS and is associated with somatization independently of depression, anxiety, and other somatic disorders21.
Dysautonomia
Several monitoring systems directed at the autonomic nervous system are being developed to better study the relationship across autonomic function, DGBI, and psychosocial interactions. Measurements include respiratory sinus arrhythmia, high-frequency heart rate variability, vagally mediated heart rate variability, vagal efficiency index, electrodermal activity, and gastric surface mapping22. Autonomic regulation has been involved in DGBI and psychosocial processes. A systematic review and meta-analysis suggest that there is a significant difference in heart rate variability when comparing IBS patients with healthy controls, although it emphasizes the need for better studies to confirm this23.
Personality dimensions
Individuals with IBS, compared with the general population, exhibit certain traits such as being less open, less agreeable, and more neurotic24. Personality in adults has been studied with the Minnesota Multiphasic Personality Inventory (MMPI), one of the most widely used psychometric tests, standardized in the U.S. population25. A study of 235 IBS patients using the MMPI showed that patients had significantly greater symptom exaggeration, lower defense scores, and higher scores for hypochondriasis, depression, hysteria, psychopathic deviation, masculinity/femininity, paranoia, psychasthenia, schizophrenia, hypomania, and social introversion compared with individuals with DGBI but without IBS26.
Abuse, early-life adversity, and post-traumatic stress
Patients with DGBI who have a history of abuse frequently seek health care services. The risk of developing IBS doubles in individuals with adverse childhood experiences, including mental illness, parental incarceration, or sexual, physical, or emotional abuse5. Early-life traumatic events lead to persistent alterations in corticotropin-releasing factor activity and poor regulation of the hypothalamic-pituitary axis, resulting in an exaggerated stress response. Up to 50% of women seen in academic gastroenterology clinics report a history of early trauma, more severe pain, and worse clinical outcomes27. Symptoms particularly associated with a history of abuse include constipation with dyssynergic defecation, chronic abdominal and pelvic pain, eating disorders, obesity, multiple DGBI, and functional somatic syndromes28.
Stress and fatigue
In the Rome Foundation Global Study, which included 54,127 patients, individuals with clinically relevant psychological distress or somatic symptoms were found to be 4.45 times more likely to have one or more DGBI29. Stress affects digestive tract physiology, the subjective experience of symptoms, health behaviors, and treatment response. Cognitive information and external stressors can influence gastrointestinal sensation, motility, and secretion through neural connections30. Stress and emotions may trigger neuroimmune or neuroendocrine reactions through the gut–brain axis, influencing GI, endocrine, and immune function. Prospective and retrospective studies show that acute or chronic stress, and increased stress levels, exacerbate IBS symptoms30,31. Imaging modalities support that acute stress and experimentally induced mood states affect neural activation in response to visceral stimuli32,33. Patients with IBS have lower stress resilience, more severe gastrointestinal symptoms, and altered cortisol responses34. Acute psychosocial stress provokes negative expectations regarding the perception of visceral pain35. Fatigue is an important element of IBS, reported in 54% of patients vs 25-30% of healthy subjects36. More than 40% of individuals with IBS have anxiety and depression, which are closely associated with fatigue37. Sleep disturbances are reported in 73% of individuals with IBS, compared with 37% of healthy controls38. A common complaint in IBS is “brain fog,” characterized by confusion, forgetfulness, poor concentration, and lack of mental clarity. Reports also describe impairments in memory, executive function, and attention, at least in a subgroup of patients39. In one study of 49 patients, significantly higher levels of fatigue, anxiety, depression, sleep disturbances, and reduced performance on psychometric tests of attention and memory were found vs a control group. Physical fatigue and anxiety were the features that best discriminated between patients and healthy controls39.
Non-pharmacological treatments for irritable bowel syndrome
Despite the multiple treatments available for IBS, a proportion of patients have persistent symptoms that do not respond to laxatives, antispasmodics, analgesics, or neuromodulators. Up to 40% of patients present with anxiety or depression, but whether these precede or are a consequence of DGBI remains debated40. Individuals with DGBI may also suffer from fibromyalgia, chronic fatigue syndrome, overactive bladder, chronic pelvic pain, and other chronic pain syndromes41. Mind-body treatments have been developed, as shown in table 1. The therapies with the strongest scientific evidence are cognitive behavioral therapy (CBT) and gut-directed hypnotherapy42. Although non-pharmacological treatments are currently recommended mainly for patients who fail medical therapy, they are increasingly being considered as early treatment options or as part of a comprehensive management plan43. When non-pharmacological therapy is applied in IBS, it targets several objectives, summarized in table 2. Such interventions can improve key components of DGBI pathophysiology, detailed in table 3.
Table 1. Classification of non-pharmacological treatments for irritable bowel syndrome
| Non-pharmacological therapies for irritable bowel syndrome |
| – Mind-body |
| • Exercise |
| • Relaxation |
| • Diaphragmatic breathing |
| • Autogenic training |
| • Meditation: mindfulness |
| – Movement with meditation |
| • Yoga |
| • Tai chi |
| • Qigong (chi-kung) |
| – Brain–gut therapies (including minimal contact approaches) |
| • Cognitive-behavioral therapy |
| • Interoceptive exposure |
| • Acceptance and commitment therapy |
| • Emotional awareness therapy |
| ⦿ Hypnotherapy |
| ⦿ Digital therapy or physical manipulation |
| ⦿ Interpersonal psychodynamic therapy |
| – Acupuncture and complementary medicine techniques |
| – Emerging therapies |
| • Vagal stimulation |
| • Virtual reality |
| • Sacral neuromodulation |
| • Biofeedback |
| • Non-invasive electrical stimulation |
| • Transcranial direct current stimulation Repetitive transcranial magnetic stimulation |
|
Adapted from Wang et al.42. |
Table 2. Objectives of non-pharmacological treatments for irritable bowel syndrome
| Conditioned physiological alterations |
| Visceral hypersensitivity |
| Anxiety and gut-specific avoidance behavior |
| Anxious traits |
| Generalized stress |
| Difficulty processing emotions |
| General interpersonal difficulties |
|
Adapted from Chey et al.43. |
Table 3. Non-pharmacological therapeutic techniques that improve the central components of gut-brain interaction disorders
| Habit reversal training |
| Cognitive techniques |
| Exposure techniques |
| Relaxation training |
| Clinical hypnosis |
| Mindfulness training |
| Emotional processing techniques |
| Interpersonal effectiveness |
|
Adapted from Chey et al.43. |
Doctor-patient relationship
A good doctor-patient relationship reduces the number of investigations, decreases health care utilization, and improves satisfaction for both the physician and the patient44. In a prospective follow-up of community individuals over 12 years, among those who had symptoms at baseline, 20% had the same symptoms, 40% had no symptoms, and 40% had different symptoms; explaining this to patients provides reassurance45. The Mexican Consensus on IBS establishes that a strong doctor–patient relationship has positive effects on global improvement, symptom improvement, severity scores, and quality of life46.
Non-pharmacological therapy for irritable bowel syndrome
Mind-body interventions
EXERCISE
According to the World Health Organization, physical activity is any skeletal muscle movement involving energy expenditure. Exercise, by contrast, is planned, structured, and repetitive physical activity. Both have beneficial effects on health. Physical activity prevents the onset of gastrointestinal symptoms in healthy individuals47. Physical activity levels are lower in individuals with IBS than in healthy controls48. Long-term exercise reduces mild anxiety and depression, increases parasympathetic activity and serotonin activity in the CNS, improves intestinal transit time, decreases bloating, and has anti-inflammatory and antioxidant effects in IBS49. Exercise also induces changes in blood flow, neuroendocrine and immune responses, and intestinal motility, while reducing stress and enhancing well-being50. Increasing physical activity in IBS patients can improve both intestinal and extraintestinal symptoms50. In a program of moderate-to-intense exercise (20-60 minutes, three to five times per week), significant improvement in symptom scores was demonstrated after 12 weeks51.
The positive impact of exercise on IBS has potential long-term benefits (> 5 years), improving GI and psychological symptoms such as fatigue, depression, and anxiety52,53. A Cochrane review included 11 randomized studies with 622 patients54. Five studies analyzed supervised physical activity, three unsupervised activity, and 3 a mixture of both. None had low risk of bias. A meta-analysis of six randomized trials showed improvement in global symptoms after physical activity, although certainty of evidence was very low. Two studies compared yoga with walking for IBS global symptoms; the meta-analysis (124 subjects) showed no inter-group differences (standardized mean difference [SMD]: –1.16; 95% CI; –3.93 to 1.62)54. Regarding quality of life and pain, the meta-analysis found no difference with physical activity vs usual care. The authors concluded that exercise may improve symptoms, but not quality of life or abdominal pain, with low certainty of evidence. Because of limited evidence, only the British Society of Gastroenterology guidelines recommend regular exercise as initial treatment with strong recommendation but low-quality evidence55. The Mexican Consensus on IBS also recommends exercise, with weak evidence and weak strength of recommendation in favor of the intervention46.
RELAXATION TECHNIQUES
Reducing hyperarousal is an essential component in the treatment of DGBI12. The trainer teaches the technique to the patient, who must then practice it at home.
DIAPHRAGMATIC BREATHING
This technique decreases vagal tone, which is often increased in IBS. It is also used in the treatment of rumination syndrome, abdominophrenic dyssynergia associated with objective bloating, and supragastric belching syndrome. In abdominophrenic dyssynergia, studies have shown improvement in both subjective and objective distension56.
AUTOGENIC TRAINING
This is a technique that can be applied by physicians or other health care personnel. After several sessions, patients can perform the treatment on their own. It can be administered according to the Schultz protocol57. Table 4 lists the exercises performed with patients. In a small study with 21 patients randomized to receive autogenic training or counseling on habits and diet, adequate improvement was achieved in 81.8% of patients in the autogenic training group vs 30% of controls57. In quality-of-life scales, the social functioning and bodily pain subscales improved significantly with autogenic training, while emotional role and general health showed a trend toward improvement in this group57.
Table 4. Autogenic training exercises
| My right (left) arm (leg) is heavy |
| My right (left) arm (leg) is warm |
| My heartbeat is calm and regular |
| I am breathing |
| My solar plexus is warm |
| My forehead is cool and clear |
| Cancellation |
|
Adapted from Shinozaki et al.57. |
MINDFULNESS (MEDITATION)
This technique is not considered a relaxation exercise, but rather a strategy to reduce vulnerability to stress. Inspired by Buddhism, it is based on observing conscious experiences, perceptions, thoughts, feelings, and sensations without judging them as good or bad, true or false58. Mindfulness for IBS focuses on reducing hyperarousal and stress to lower the risk of symptomatic flare-ups. It emphasizes remaining calm in stressful moments and accepting pain as inevitable, which can reduce both suffering and pain. It targets the elimination of unhelpful psychosocial processes such as rumination, worry, and poor emotional regulation, thereby improving symptoms, psychological processes, and quality of life12. In randomized controlled trials mindfulness therapy has been shown to improve constipation, diarrhea, bloating, and anxiety related to the digestive tract. It may reduce visceral sensitivity and improve cognitive appraisal of symptoms. Meditation can also modify the unpleasant experience associated with symptoms by changing the perception of threat they represent59. In a prospective, non-randomized study of 93 IBS patients (Rome III criteria), mindfulness therapy improved IBS-related quality of life and GI anxiety, but not specific symptom severity60. In a study with 75 patients randomized to mindfulness or a support group, a clinically significant improvement in symptom severity was reported in the mindfulness group61. At treatment end, there were no significant differences in psychological distress, quality of life, or visceral anxiety between groups, but at 3 months, significant improvements were observed in the mindfulness group61. In another trial, 90 IBS patients (Rome III criteria) were randomized to mindfulness-based stress reduction therapy or waitlist control30. Both groups improved in IBS symptoms, but improvement was significantly greater and clinically meaningful in the mindfulness group. At 6 months, mindfulness patients maintained clinical benefit vs controls, though differences were not statistically significant. A more recent study by Naliboff et al.59 employed the University of Massachusetts mindfulness-based stress reduction protocol: 8 2-hour weekly sessions plus a 4-hour retreat. Classes were group-based (8-12 participants). GI symptom response was achieved in 71% of participants59. A meta-analysis considered mindfulness therapy effective for IBS treatment56.
Movement with meditation
YOGA, TAI CHI, AND QIGONG (CHI-KUNG)
Both yoga and qigong (also called chi-kung) are ancient techniques that likely originated in India (yoga) and were later exported to and developed in China (qigong). They are based on the concept of prana (qi), the energy that flows through the nadis (meridians). The objectives of yoga include accumulating more prana through control of breathing and postures (asanas). Qigong develops symmetrical movements to balance both sides of the body, incorporating breathing techniques in combination with movements, and is a practice focused inward. Tai chi, meanwhile, is a martial art originating in the 17th century with the aim of maintaining center and balance while repelling aggression. Movements have martial purposes, and breathing is not necessarily coordinated with them. Tai chi is linked to interaction with others. Yoga is thought to correct reduced parasympathetic activity in stress-related disorders62. A systematic review of 6 controlled studies of yoga in IBS showed that yoga was superior to conventional care, with statistically significant reductions in intestinal symptoms, symptom severity, and physical functioning. No differences were found between yoga and exercise. Yoga was safe, with no adverse effects. However, the studies were heterogeneous and had methodological limitations63. A Cochrane meta-analysis (124 subjects) found no differences between walking exercise and yoga in terms of improvement of global IBS symptoms (SMD, –1.16; 95% CI, –3.93 to 1.62)54. Similarly, 2 studies comparing yoga with pharmacologic treatments found no differences, though certainty of evidence was low and risk of bias high. One study compared yoga with dietary intervention and reported improvement with both methods, with no differences between them. Quality of life improved more with yoga compared with walking. No effect was found on abdominal pain54. Another study showed that Baduanjin qigong in older adults with constipation-predominant IBS (IBS-C) was superior when combined with tegaserod vs tegaserod alone64. Two studies have described the effects of tai chi in IBS patients. Both demonstrated improvement in therapeutic effect, symptoms, and stool characteristics vs a mosapride-treated group (p < 0.05). Another study observed improvement in anxiety and depression scores in the intervention group compared with usual treatment (p < 0.05)42.
Brain–gut therapies
COGNITIVE-BEHAVIORAL APPROACHES
Behavioral therapies used for DGBI are short-term, non-pharmacological treatments that aim to improve GI symptoms by addressing psychological comorbidity. They do not focus on a specific gastrointestinal symptom but rather on improving gut–brain interaction, incorporating techniques that modify psychosocial and psychological processes42. B.F. Skinner and J. Wolpe were pioneers of behavioral therapies in the 1950s. They were based on the idea that changing behavior modifies the perception of emotions and cognition. Cognitive psychotherapy focuses on changing concepts, thereby altering emotions and behaviors. Later, the concepts of cognitive therapy and behavioral therapy were merged as cognitive-behavioral therapy (CBT)65. CBT has been applied in post-traumatic stress disorder, panic disorder, phobias, and social anxiety disorder65.
Techniques based on exposure therapy are grounded in the idea that fear is represented by cognitive structures that contain information about the feared stimulus, the fear responses, and the meaning of both (e.g., lion = danger, therefore increased heart rate and acute myocardial infarction). When encountering a stimulus resembling the feared one, these cognitive fear structures are triggered, becoming pathological when disproportionate to reality or occurring in normal situations. Exposure therapy works by exposing the patient to the feared stimulus to change the response65.
Cognitive therapy is based on Beck’s tripartite model, which proposes that thoughts, feelings, and behaviors are interrelated. If maladaptive thoughts are modified, feelings and behaviors also change. Psychoeducation about distorted thinking (e.g., all-or-nothing thinking, jumping to conclusions, disqualifying the positive) and cognitive restructuring are integral components.
COGNITIVE-BEHAVIORAL THERAPY (CBT)
CBT is one of the best-studied behavioral therapies in DGBI. Its effectiveness has been demonstrated in IBS, non-cardiac chest pain, and functional dyspepsia. Symptom improvement appears to be due not to changes in generalized stress or anxiety, but rather to reduced avoidance behavior and altered illness-related concepts12. CBT targets the cognitive, affective, and behavioral processes that trigger or exacerbate digestive symptoms. Patients with DGBI often display hypervigilance, excessive fear and discomfort about symptoms, and excessive avoidance behaviors13. Predictors of treatment response in CBT include the degree of gastrointestinal-specific anxiety, alexithymia, anxious traits, anxiety sensitivity, and coping styles66.
INTEROCEPTIVE EXPOSURE
This approach is useful in individuals with strong avoidance behaviors. Patients with somatic symptoms often develop hypersensitivity to bodily sensations and a fear–avoidance pattern that, in turn, increases symptom perception67. Treatment involves exercises that reduce fear and hypervigilance by exposing patients to IBS-related sensations: eating foods believed to trigger symptoms, wearing tight clothing, reducing bathroom visits, exercising, etc. This therapy can be delivered in groups or online and has been shown to improve symptoms and quality of life68. In a non-randomized, uncontrolled study of group-delivered exposure-based CBT, symptom severity decreased by 34% after treatment (p < 0.001), and IBS-related quality of life improved by 68.2% (p < 0.001)69. In a study of 309 participants, CBT alone was compared with CBT plus interoceptive exposure. After 10 weeks, the latter group showed significantly greater treatment response, leading the authors to conclude that exposure therapy has incremental effects over other CBT components68.
ACCEPTANCE AND COMMITMENT THERAPY (ACT)
ACT includes emerging behavioral therapies that incorporate psychological flexibility techniques and promote behaviors aligned with patient values42. A quasi-experimental study demonstrated improvement in depression and psychological capital in individuals with IBS.
EMOTIONAL AWARENESS TRAINING THERAPY
Individuals with IBS often present with alexithymia, defined as the difficulty in experiencing, expressing, and describing emotional responses. In a study of IBS patients diagnosed according to Rome III criteria, the Toronto Alexithymia Scalewas applied, which evaluates difficulty identifying feelings, difficulty describing feelings, and externally oriented thinking21. Of the 100 patients evaluated, 70 met the inclusion criteria and 60 completed the study. Patients with IBS had higher levels of alexithymia compared with a healthy population. Thirty patients received medical treatment alone, while another 30 received optimal medical therapy combined with emotional awareness training therapy. The group on emotional awareness training therapy showed a 54% reduction in pain severity score vs 36% in the optimal medical treatment group. Pain frequency decreased by 59% in the combined therapy group and 34% in the optimal medical treatment group, both with significant differences (p = 0.015 and p < 0.005, respectively). Alexithymia, when analyzed as a covariate, did not have a significant effect on the response to pain intensity or pain frequency. Nevertheless, further studies are needed to confirm the efficacy of this treatment.
EXPRESSION THERAPIES
A randomized trial42 showed that expressive writing therapy, in which patients wrote about their deep thoughts regarding IBS in four sessions, improved pain and reduced health care utilization compared with controls. Another randomized trial of emotional awareness and expression therapy, focused on emotional avoidance in patients with a history of trauma and emotional conflicts, demonstrated reduced IBS symptom severity and improved quality of life42.
GUT-DIRECTED HYPNOTHERAPY
This is a hypnotherapy approach administered by a trained clinician who places patients in a state of alertness and focus that increases receptivity to post-hypnotic suggestions. Evidence shows that hypnosis can normalize visceral sensitivity and motility70. Gut-directed hypnotherapy is administered in multiple sessions in which patients are guided into deep relaxation, with attention focused on enhancing acceptance of therapeutic suggestions. The Manchester and North Carolina protocols are the most widely used. In Manchester, this therapy has been practiced since 1984, when it was shown to improve pain, bloating, bowel disturbance, and overall well-being compared with placebo and supportive therapy. Treatment consists of 12 weekly sessions. Techniques include developing the ability to control the bowel, placing a warm hand over the abdomen, and visualizing a normal intestine. Post-hypnotic suggestions are also provided71. In North Carolina, a 7-session, twice-weekly protocol is used. Researchers tried to measure parasympathetic effects in responders (heart rate, systolic/diastolic blood pressure, temperature, skin conductance, skeletal muscle tension), but no measurable changes were documented. Nonetheless, four out of five patients respond to treatment72. Although meta-analyses confirm the efficacy profile of hypnotherapy for IBS symptom improvement73, protocols vary: some use gut-directed hypnotherapy, while others combine hypnotherapy with CBT or other integrated treatments74. Session duration also varies widely, from 3 to 16 sessions, lasting 150 to 720 minutes. However, in reviews of non-pharmacological treatments, hypnotherapy is considered effective75. No significant differences have been found between individual and group therapy, suggesting that group therapy may be a cost-effective strategy76. The most recent meta-analysis concludes that face-to-face gut-directed hypnotherapy has evidence of efficacy77.
DIGITAL THERAPY OR PHYSICAL MANIPULATION
Osteopathic physicians have in their therapeutic arsenal osteopathic manipulative treatments, which consist of applying gentle pressure on tissues. A 2014 systematic review of 5 studies with 204 patients showed significant short-term benefits vs sham treatment or usual care78. However, as with acupuncture, the practitioners delivering these treatments use different, poorly standardized approaches. The objectives are unclear and symptom rating scales are poorly defined, making these findings difficult to interpret.
PSYCHODYNAMIC INTERPERSONAL THERAPY
This therapy is delivered by a highly trained psychotherapist and is based on the principle that a strong, collaborative, and trusting relationship between patient and therapist is itself a mechanism of change. Through this relationship, the patient may repair negative emotions contributing to DGBI. It is recommended for patients with severe, persistent symptoms, in whom interpersonal difficulties and illness have become central to their life79. Meta-analyses show the effectiveness of psychodynamic interpersonal therapy in functional somatic disorders80. Randomized controlled trials have been conducted in IBS and functional dyspepsia81,82. This therapy is particularly useful in cases with a history of trauma or early-life adversity83.
MINIMAL-CONTACT AND DIGITAL THERAPIES
IBS treatments can be costly and not widely available, leading to the development of minimal-contact methods. A systematic review of minimal-contact psychological therapies for IBS found reductions in symptom severity and improvements in quality of life84. A systematic review and meta-analysis of 10 RCTs including 886 patients showed that, compared with controls, minimal-contact interventions had a moderate effect on symptom improvement and a large effect on quality of life. Online interventions were more effective than other formats85. Telephone CBT and the Mahana IBS program, both showing significant improvements in symptom severity, quality of life, and mood at 12 months. Compared with usual care, symptoms were significantly reduced (p < 0.001 for telephone CBT, p = 0.002 for Mahana IBS), with benefits sustained at 24 months86. Zemedy, a CBT-based digital application, in an RCT managed to reduce IBS symptom severity and improved quality of life compared with a waitlist group (p < 0.001)87.Digital hypnotherapy has also been studied. In a retrospective evaluation of 190 self-diagnosed IBS patients using the Nerva app, a 64% positive response (defined as > 30% reduction in abdominal pain) was observed after 4 weeks88. Another RCT found that digital hypnotherapy was slightly less effective than face-to-face therapy for the primary endpoint (≥ 50-point reduction in IBS severity score: 65% vs. 76%), but access was greater89.
Virtual yoga has been tested in RCTs and shown to be safe and feasible, with a positive impact on symptom severity within groups (baseline vs. post-treatment), but not across groups. Significant between-group differences were found in quality of life, fatigue, and perceived stress (favoring yoga), but not in symptom severity90.
A systematic review identified 929 studies, of which 13 high-quality studies (21,510 participants) were included, focusing on education, diet, brain–gut behavioral tools, psychological support, health monitoring, and community engagement. Most digital tools were self-directed and showed significant improvements in most outcomes assessed91.
Acupuncture and complementary medicine techniques
TRADITIONAL CHINESE ACUPUNCTURE
Acupuncture is a Chinese treatment with more than 2,000 years of history, based on the insertion of thin needles into specific acupoints along the 12 meridians or energy channels, with the intention of balancing energy flow in the body. This technique has been studied not only in humans but also in animal models, aiming to improve visceral hypersensitivity, inflammation, and central sensitization, to modulate the serotonergic response, regulate gastrointestinal motility, and reduce the overexpression of substance P and vasoactive intestinal peptide in IBS-C populations42. A 2006 Cochrane review of 6 individual studies showed that acupuncture administered at true points was no different from sham acupuncture, but it was superior to herbal medicine, and superior in combination with psychotherapy compared with psychotherapy alone92. However, study quality was low and heterogeneity high, preventing firm conclusions. A 2014 meta-analysis of 6 RCTs reported that 1 was positive and 5 were negative, but overall, the combined effect was positive (OR, 1.75; 95% CI, 1.24-2.46)93. Other meta-analyses suggest that both acupuncture and sham acupuncture outperform pharmacological treatments, with fewer side effects94. A more recent systematic review and meta-analysis assessed studies comparing acupuncture with sham acupuncture, usual care, pharmacologic treatments, and other interventions, focusing on quality of life in IBS patients95. Secondary outcomes included abdominal pain and severity scores. Fourteen studies with 2,038 participants were included. Acupuncture significantly improved quality of life vs usual care (mean difference [MD], 6.62; 95% CI, 2.30-10.94; p < 0.001; I² = 72.45%). Acupuncture was also superior in improving symptom severity (MD, –46.58; 95% CI, –91.49 to –1.68; p < 0.001; I² = 90.76%). However, it did not improve abdominal pain. Adverse events were rare. In reviews of non-pharmacological IBS treatments, the utility of acupuncture remains unclear—there may be a modest positive effect with low risk of adverse events. Because of conflicting results and variability in protocols across practitioners, standardization attempts have been made. Using the Delphi methodology, experts in acupuncture reviewed the English literature and established recommendations for session frequency, duration, and treatment length to improve efficacy96.
ELECTROACUPUNCTURE AND MOXIBUSTION
Electroacupuncture involves applying small electrical currents through acupuncture needles to enhance therapeutic effect. While RCTs have been conducted in constipation, its utility in IBS remains unclear42. A recent meta-analysis reported positive outcomes with acupuncture, electroacupuncture, and moxibustion (burning the Artemisia plant at specific body sites) for diarrhea-predominant IBS (IBS-D), either alone or combined with other treatments. However, the authors noted that more high-quality studies are needed97.
Emerging therapies
VIRTUAL REALITY
Virtual reality (VR) is a 3D computer-generated environment designed to make patients feel immersed within it. Sensors track head position to adjust the environment, and patients typically use headphones. VR has been used in acute and chronic pain management through distraction, decreased sensitivity, and mood alteration associated with symptoms. It is thought to reduce pain by stimulating the visual cortex and other senses, acting as a distractor that limits the perception of painful stimuli and inducing “attentional blindness”, which decreases the ability to focus on pain98. Neuroimaging shows that VR affects pain processing in the sensory and insular cortices, suggesting that it reduces both intensity and emotional response to pain. Comparisons with opioids using functional MRI indicate that VR has similar effects in blocking pain99. In IBS, VR is being studied as a combination of education, CBT techniques, and exposure therapy in an immersive environment. Future studies will clarify its potential benefit100. A multidisciplinary group developed a VR program for IBS including essential CBT components: psychoeducation, relaxation strategies, cognitive restructuring, problem-solving skills, and exposure techniques. Programs last 8 weeks, with daily 5-20 minute sessions, reinforced by daily messages and CBT exercises delivered through a mobile or computer app101. This novel approach is currently in phase I trials102.
SACRAL NEUROMODULATION
Sacral neuromodulation has been applied in a small number of patients with diarrhea-predominant IBS (IBS-D) or mixed IBS (IBS-M). The rationale was that IBS-D symptoms resemble those of fecal incontinence. Later studies showed that patients with constipation-predominant IBS (IBS-C) respond less favorably than those with diarrhea103. In a prospective cohort of IBS patients treated with sacral neuromodulation, outcomes were analyzed at 1, 3, 5, and 10 years104. All patients had IBS-D or IBS-M and were refractory to usual treatment. After 2-3 weeks of percutaneous stimulation, patients showing ≥ 30% improvement proceeded to permanent implantation. Of 36 implanted patients, 23 were analyzed at 5 years and 13 at 10 years. The GSRS-IBS symptom scale showed significant reductions at 5 years (p < 0.0001) and 10 years (p = 0.0007). Five patients required device removal due to adverse events104.
BIOFEEDBACK
A Cochrane review of RCTs involving 300 patients analyzed multiple biofeedback modalities: thermal (skin temperature in 4 studies), rectosigmoid (rectal manometry/barostat in 1 study), pulse variability (pulse oximetry in 2 studies), and electrocutaneous biofeedback (2 studies). All aimed to teach patients to control bodily processes such as heart rate and breathing. The review found high or unclear bias in all studies, with uncertain utility of biofeedback for IBS105. Heart rate variability (HRV) biofeedback is thought to regulate the autonomic nervous system, reduce stress, and decrease psychological distress. It increases vagal tone and vagal flexibility by using vagally mediated respiratory sinus arrhythmia106. This therapy involves slowing respiratory rate to 4.5-7.2 cycles per minute to enhance HRV. In a small study of 29 patients, 3 sessions were delivered over 24 days, with patients instructed to practice at home for 5 minutes, 3 times daily. Autonomic activity was measured at rest, during a mental task, and during recovery. Results showed that HRV biofeedback reduced psychological distress and hopelessness, and decreased sympathetic reactivity during mental stress107. These findings still require confirmation in larger RCTs.
NONINVASIVE ELECTRICAL STIMULATION
Neuromodulation can also be achieved by stimulating superficial nerves beneath the skin using transcutaneous electrical pulses108. Transcutaneous electrical acustimulation (TEA): surface electrodes placed on acupuncture points near a peripheral nerve. Transcutaneous tibial nerve stimulation (TNS): electrodes applied on the skin above the ankle. When needles are inserted, it is called percutaneous tibial nerve stimulation (pTNS). Transcutaneous auricular vagal nerve stimulation (taVNS): electrodes placed on the ear in areas innervated exclusively by the vagus nerve (cymba conchae). Percutaneous auricular vagal nerve stimulation (paVNS): miniature electrodes placed in auricular regions with vagal innervation. Interferential current (IFC): originally used in genitourinary dysfunction, it is produced by intersecting 2 diagonal, opposing currents that generate a medium-frequency current capable of penetrating nerve fibers in the target organs109.
In an RCT including 52 IBS-C patients, TEA at ST36 and PC6 improved constipation and abdominal pain. After 4 weeks of daily treatment, weekly spontaneous bowel movements increased from 2.3 to 3.5, and abdominal pain decreased by 42%110. In 42 IBS-D patients, TEA at LI4 (Hegu) and ST36 improved quality of life and abdominal pain, though no significant changes were observed in plasma norepinephrine, pancreatic polypeptide, or cytokines, so autonomic or inflammatory modulation could not be confirmed111. In IBS-C patients, taVNS increased weekly spontaneous bowel movements and reduced abdominal pain112. It also improved rectal sensation (anorectal manometry), reduced serum proinflammatory cytokines, and increased vagal activity (measured by HRV)112. In 60 adolescents with IBS, paVNS significantly reduced abdominal pain vs sham treatment over 3 weeks, with sustained effects at 9.2 weeks113. A different RCT with 50 adolescents showed ≥ 30% reduction in abdominal pain in 59% of paVNS-treated patients vs 25% in controls114. In an RCT with 58 adults, IFC and sham stimulation both improved symptoms, but active IFC continued to reduce severity and improve visual analog scores more than sham115.
TRANSCRANIAL DIRECT CURRENT STIMULATION (TDCS) AND REPETITIVE TRANSCRANIAL MAGNETIC STIMULATION (RTMS)
The cerebellum regulates not only movement but also interacts with nociceptive pathways, pain anticipation, and emotional responses to pain. Certain cerebellar regions remain persistently activated during pain perception and anticipation116. These areas are connected with both the sensory–discriminative and affective–motivational dimensions of pain. Studies in IBS patients have demonstrated increased cerebellar activation. Noninvasive brain stimulation modalities targeting these areas have been tested: tDCS: application of weak direct current. Anodal stimulation (positive) increases cortical excitability, whereas cathodal (negative) decreases it117. Pain sensitivity studies show that anodal tDCS enhances endogenous pain inhibition118. In addition, rTMS: application of high- or low-frequency magnetic fields to cortical regions. Low frequencies inhibit, while high frequencies excite neural activity119. Future studies will clarify whether these neuromodulation techniques are useful non-pharmacological treatments for IBS pain.
The Mexican Association of Gastroenterology has recently published (online ahead of print), in collaboration with the Mexican Association of Neurogastroenterology and Motility, clinical best practice recommendations for the use of neuromodulators in gastroenterology. In addition to the indications and correct use of neuromodulators, these guidelines also include non-pharmacological treatments, which encompass psychological therapies as well as external and digital devices120.
Conclusions
Non-pharmacological treatment of IBS does not invalidate traditional optimal medical therapy; rather, it represents a useful alternative. The clinician who faces the daily suffering of IBS patients has at their disposal tools that can improve patient outcomes. The main challenge lies in having a multidisciplinary team capable of professionally applying these treatments. Regarding minimal-contact therapy, the greatest barrier is language, since most applications are available only in English. However, this represents an area of opportunity for Spanish-speaking developers. Fig. 1 suggests the non-pharmacological techniques that may be useful according to the specific characteristics of each patient.
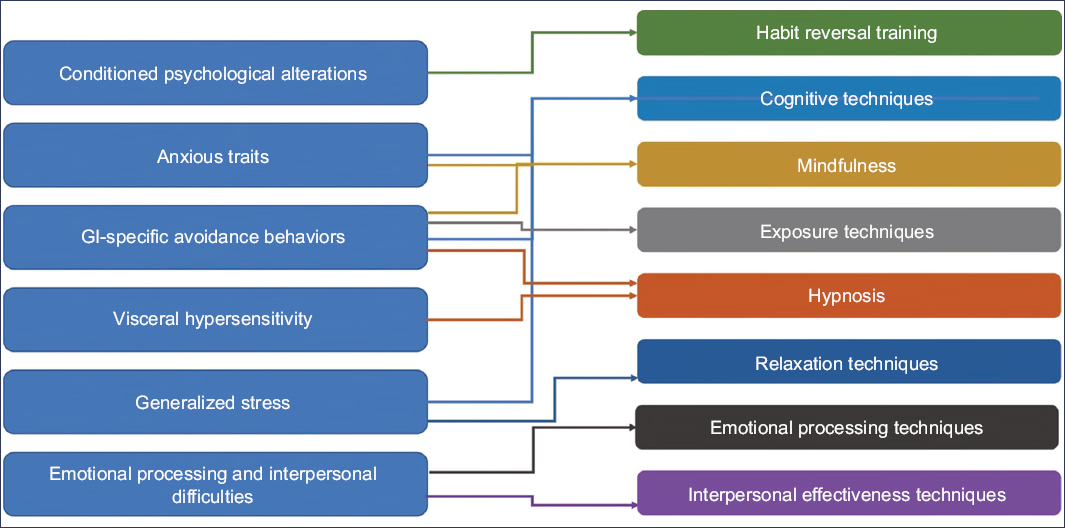
Figure 1. Non-pharmacological techniques of usefulness based on the pathophysiological characteristics of each patient (modified from Wang et al.42).
Funding
The authors declare that they received no funding for this work.
Conflicts of interest
The authors declared no conflicts of interest whatsoever.
Ethical responsibilities
Protection of persons and animals: The authors declare that no experiments were conducted on humans or animals for this research.
Confidentiality of data: The authors declare that no patient data appear in this article.
Right to privacy and informed consent: The authors declare that no patient data appear in this article.