Introduction
The diagnosis of irritable bowel syndrome (IBS) is established based on symptoms, which include abdominal pain associated with a change in stool form or frequency, with the Bristol Stool Scale being very useful for characterizing stool types1 (Table 1). Symptoms usually vary in intensity and frequency, and may worsen in stressful situations. Changes in bowel habits allow IBS to be subclassified 4 four subtypes: with diarrhea predominance (IBS-D), with constipation predominance (IBS-C), mixed type (IBS-M), alternating between diarrhea and constipation, and unclassified (IBS-U) when it does not meet the criteria of the previous three. Recently published information indicates that the prevalence of IBS-C, IBS-D, and IBS-M is similar, with IBS-M being the least common2.
Table 1. Rome IV diagnostic criteria for irritable bowel syndrome
| IBS-C | IBS-D | IBS-M | IBS-U |
|---|---|---|---|
| > 25% of bowel movements hard (Bristol 1-2) and < 25% loose (Bristol 6-7). At least 1 daily bowel movement altered in form. Constipation predominance is established when evacuation occurs without the use of medications. | > 25% of bowel movements loose (Bristol 6-7) and < 25% hard (Bristol 1-2). At least 1 daily bowel movement altered in form. Diarrhea predominance is established when evacuation occurs without the use of drugs. | > 25% of bowel movements loose (Bristol 6-7) and > 25% hard (Bristol 1-2). | Although patients meet diagnostic criteria for IBS, their bowel pattern cannot be categorized into any of the other 3 types. |
|
IBS-D: irritable bowel syndrome with diarrhea predominance; IBS-C: irritable bowel syndrome with constipation predominance; IBS-M: irritable bowel syndrome with mixed pattern; IBS-U: unclassified irritable bowel syndrome. |
|||
Emerging phenotypes and endophenotypes
Endophenotypes in IBS are specific subgroups of symptoms or characteristics present in the patient that can help identify different forms of the disease and improve individualized treatment. These endophenotypes contribute to a better understanding of how IBS manifests in different people and how to offer the best treatment for each case. Among the endophenotypes or pathophysiological mechanisms are central nervous system hypervigilance, psychosocial factors, genetic predisposition, and some mechanisms directly involved in the GI tract. Although IBS is often considered a disorder of the gut–brain axis, it is important to note that intestinal mechanisms exist that can be corrected without using centrally acting neuromodulators. Hypnotherapy, psychotherapy, and central neuromodulation may be useful in selected patients. However, it is essential to identify intestinal pathophysiological mechanisms to individualize treatment. In addition to the already described mechanisms present in IBS, there is evidence that the intestine may suffer direct damage from products of food digestion, neurotransmitters, prior enteritis, the microbiome, immune activation in the mucosa, and increased intestinal permeability3. These factors trigger intestinal motility disorders, altered sensitivity, and defecation disorders.
Therefore, the pathophysiological subgroups of importance in IBS include defecatory disorders, abnormal colonic transit, bile acid diarrhea, colonic and rectal hypersensitivity, disaccharidase deficiency, food-induced local immune-mediated reactions, and microbiota alteration. Numerous studies have been conducted to better understand pathophysiology, and tests have also been developed to facilitate the identification of mechanisms that produce symptoms in patients.
Defecation disorders
Defecation disorders mimic the symptoms of IBS-C, including incomplete emptying of the left colon leading to abdominal distension, abdominal pain, and constipation. Defecation disorders may result from delayed colonic transit, particularly in the left colon4. In clinical practice, 2 subtypes of pelvic floor dysfunction can be identified: spastic evacuation disorders, in which the puborectalis muscle is spastic (dyssynergia)5, or inefficient relaxation of the anal sphincter (anismus). A second category represents a flaccidity disorder, particularly in perineal descent syndrome6, or Ehlers-Danlos syndrome of the vascular or hypermobility type, with loss of connective tissue support in the perineum7,8. The diagnosis of these defecatory disorders is established by anorectal manometry with balloon expulsion testing, and the most useful parameter is increased resting anal sphincter pressure, as well as a negative rectoanal pressure index and prolonged balloon expulsion time adjusted for sex and age.
Motor dysfunction
Motor dysfunction can be demonstrated through noninvasive studies such as the use of radiopaque markers or scintigraphy. Colonic transit studies are not indicated as an initial approach but may be performed in cases of poor response to first-line treatments, such as loperamide in IBS-D or fiber and osmotic laxatives in IBS-C. Measurement of colonic transit is a diagnostic biomarker that excludes rectal defecatory disorders in patients with evidence of slow colonic transit4. However, colonic transit is not useful to differentiate IBS-D from functional diarrhea or IBS-C from functional constipation9. In patients with accelerated colonic transit and IBS-D, the purpose of conducting this study is to confirm the severity of diarrhea or the impact of pharmacologic treatment choice, or to add a second-line drug such as a 5-hydroxytryptamine (5-HT3) antagonist to a first-line therapy such as loperamide. In the case of delayed colonic transit, colonic transit testing may indicate the need to add a secretagogue to a first-line osmotic laxative for constipation in IBS-C.
Visceral hypersensitivity
Furthermore, in IBS, hypersensitivity or hypervigilance due to visceral signaling may also be present. In a classic study by Ritchie10, IBS patients exhibited rectal sensitivity to balloon distension and experienced pain with smaller distension volumes than healthy controls. Later studies demonstrated 2 distinct types of rectal sensitivity: hypersensitivity or hyperalgesia11. Thus, some patients experience pain or other sensations with less balloon distension volume, while those with lower sensitivity thresholds exhibit discomfort or hyperalgesia related to hypervigilance or altered regulation of afferent visceral signaling. Importantly, pain scores reported by patients are subjective and influenced by psychosocial conditions12. Molecular studies recording calcium activity in rectal biopsies have shown increased excitability of submucosal neurons in response to agonists of pronociceptive transient receptor potential (TRP) channels (vanilloid TRPV1, TRPV4, and ankyrin TRPA1)13. This information demonstrates that direct intervention in peripheral mechanisms involved in pain signaling may represent a therapeutic target when associated with visceral hypervigilance.
Bile acid malabsorption
In one study, 1 in 4 patients with IBS-D presented diarrhea due to bile acid malabsorption14. Approximately 90-95% of bile acids are reabsorbed in the terminal ileum via the apical sodium-dependent bile acid transporter. These bile acids enter the enterohepatic cycle, while the remaining 5-10% pass into the colon, where they increase permeability through their detergent effect. Once in the colon, primary bile acids are deconjugated by removal of glycine and taurine and are converted into secondary bile acids through epimerization by the colonic microbiota. The main secondary bile acids are lithocholic acid, deoxycholic acid, and ursodeoxycholic acid. In the colon, bile acids increase secretion, enhance mucosal permeability, and stimulate motility (high-amplitude colonic contractions)15. Currently, there are 3 valid biochemical parameters for the diagnosis of bile acid diarrhea16: quantification of 48-hour fecal bile acids, determination of primary bile acids in stool, and fasting serum C4 (collected before 9:00 a.m.). An additional method available in some countries is scintigraphy with selenium-75-labeled tauroselcholic acid (75SeHCAT), assessing retention after 7 days.
The current approach to IBS relies on establishing diagnosis based on symptoms. Therefore, in the absence of widely available and inexpensive screening tests, patients with bile acid diarrhea are included within IBS-D or functional diarrhea. With the introduction and availability of simple serologic and stool tests, patients with bile acid diarrhea should be excluded from the diagnosis of IBS-D.
Poor digestion or malabsorption of carbohydrates
The normal small intestine absorbs monosaccharides and disaccharides in the presence of disaccharidases in sufficient amounts; generally, these are absorbed in the first 2 meters of the small intestine17, with an equal amount of monosaccharide absorption from the intestinal lumen. Monosaccharides are transported by mediated mechanisms across the brush border of enterocytes, and slightly > 50% of these transporters are sodium-dependent. Any carbohydrate that is poorly digested or malabsorbed and reaches the colon is metabolized by colonic bacteria, leading to the production of gas, carbon dioxide, and water, resulting in an increased osmotic load that produces diarrhea. In fact, 25% to 75% of patients with disaccharidase deficiency meet the criteria for IBS18.
It is estimated that 65% of the world’s population has a reduced ability to digest lactose after childhood19. The highest prevalence is in Southeast Asia and South Africa, and the lowest in the Mediterranean coast and northern latitudes. Of note, when lactose intake is limited to 240 mL of milk or its daily equivalent, symptoms are usually mild, and exogenous lactase supplementation is not necessary20.
Sucrase-isomaltase deficiency
According to recent literature, sucrase-isomaltase deficiency has been identified in adults with IBS-D symptoms. This condition is frequently observed in pediatric patients. Four genetic mutations in the sucrase or isomaltase domains have been found in most of the common nucleotide changes in children with congenital sucrase-isomaltase deficiency21. In adults, the same 4 mutations in the sucrase or isomaltase gene have been identified. Sucrase-isomaltase deficiency is more prevalent in IBS patients vs controls, as demonstrated in one study in which 2.1% of IBS subjects presented the deficiency vs 1.2% of controls22, and in another similar study showing the deficiency in 4% of IBS patients and 2.8% of controls23.
Barrier dysfunction
Several studies have documented increased intestinal and colonic permeability in patients with IBS24, which predisposes to immune activation or inflammation25. A systematic review identified that intestinal permeability is increased, vs controls, in patients with IBS-D (9/13 studies) or post-infectious IBS (PI-IBS-) (4/4 studies), but this permeability was present only in a minority of patients with IBS-C (2/7 studies). Furthermore, there is a positive association between loss of barrier function and symptoms such as abdominal pain and changes in bowel habits26. Increased permeability occurs particularly in patients with bile acid diarrhea, in whom permeability is increased in IBS-D27. This increased permeability may be associated with immune or mast cell activation28.
Immune activation
Multiple lines of research have demonstrated mucosal immune activation in IBS. An increased number of B cells and plasma cells are observed in proximity to mast cells in the intestinal mucosa, related to adaptive immune activation in IBS, without an increase in serum immunoglobulin G (IgG), in contrast with increased luminal IgG29. In addition, there is evidence of increased release of nociceptive mediators by immune cells and the intestinal epithelium, leading to heightened excitability of pronociceptive neuronal receptors and visceral hypersensitivity. The relationship between mucosal inflammation or immune activation and IBS symptoms or subgroups has been studied. Evidence of immune activation in the rectum and left colon has been documented, although no association with symptoms or predominant intestinal disorder has been found30. In one study31 of colonic mucosal biopsies in IBS patients (30 women with IBS-C, and 31 women and 13 men with IBS-D), there were no differences in the expression of 181 genes in the ascending colon and 199 genes in the rectosigmoid. Most were overexpressed genes in IBS-D, with functions in the activation of inflammatory genes, TRPV1 (visceral hypersensitivity), and neurotransmitters/receptors (specifically purinergic, gamma-aminobutyric acid, and cannabinoid). Despite differences in gene expression in ascending colon and rectosigmoid mucosa in IBS-C and IBS-D, the diversity of gene overexpression related to immune functions, receptors, transmitters, ion channels, and transporters was similar across both subgroups. Conversely, there was a reduction in the expression of peptidase inhibitor genes PI15 and PI16, which inhibit proteases, in IBS-D, suggesting mucosal vulnerability to the effects of proteases (e.g., pancreatic or bacterial) in IBS-D31. Differential immune activation in ascending colon mucosa biopsies from 11 patients with bile acid diarrhea and 33 IBS-D controls showed greater activation in bile acid diarrhea32. Minimal differences in ileal mucosa biopsies between patients with IBS-C, IBS-D, and healthy subjects have been found33. However, extensive studies using jejunal mucosa from IBS patients have found aberrant immune responses, increased humoral immunity, molecular and functional alterations of the intestinal epithelial barrier, altered bile acid metabolism, proximity of plasma cells to nerves, mast cell and protease activation, and neuropeptide signaling with dysbiosis, all of which may relate to the origin of symptoms in IBS patients. This information suggests the role of the small intestine in IBS pathophysiology, particularly IBS-D34.
Chemical production during immune activation
Various intestinal factors, including bile acids, short-chain fatty acids, mucosal barrier proteins, histamine, proteases, tryptase, enteroendocrine cell products, and mucosal messenger RNA, are altered and may play an important role in IBS, especially IBS-D. Immune mediators, particularly those related to mast cells, may directly activate or sensitize pain-transmitting nerves, resulting in increased signaling and abdominal pain. Mechanisms of visceral hypersensitivity include histamine, serotonin, proteases, and nerve growth factor, which are present in the mucosa of IBS patients. Histamine acts on H1 receptors, sensitizing TRPV1, TRPA1, and TRPV4 channels35. Histamine and serotonin increase membrane expression and translocation in nociceptors, causing neuronal hypersensitivity36. Trypsin and other mucosal proteases cause endocytosis, mediating afferent hyperexcitability through TRP channel sensitization. Increased mast cell–derived nerve growth factor raises nerve fiber density, while increased brain-derived neurotrophic factor promotes greater nerve development37.
Microbiome and IBS
On the other hand, the microbiome—which comprises a healthy intestinal microbial community—is diverse, stable, resistant, and resilient. Intestinal dysbiosis occurs when the composition and function of the intestinal microbiome are altered, potentially by pathobionts, commensals, or decreased diversity. Infections, inflammation, diet, xenobiotics, genetics, circadian rhythm disruption, maternal diet, pregnancy, and physical injury may contribute to dysbiosis38. A systematic review and meta-analysis38 found no characteristic microbiota associated with IBS, nor differences between microbiomes in IBS-D and IBS-C; however, the quality of evidence was low. Other longitudinal microbiome studies38, involving 30 individuals with IBS-C, IBS-D, and healthy controls, showed significant overlap but differences in diversity. Notably, 6 patients with IBS-D and 6 patients with IBS-C developed symptoms with different types of microbiota. The clinical significance of diagnosis and treatment in microbiome characterization in IBS remains unclear39.
Clinical relevance of classification (Fig. 1)
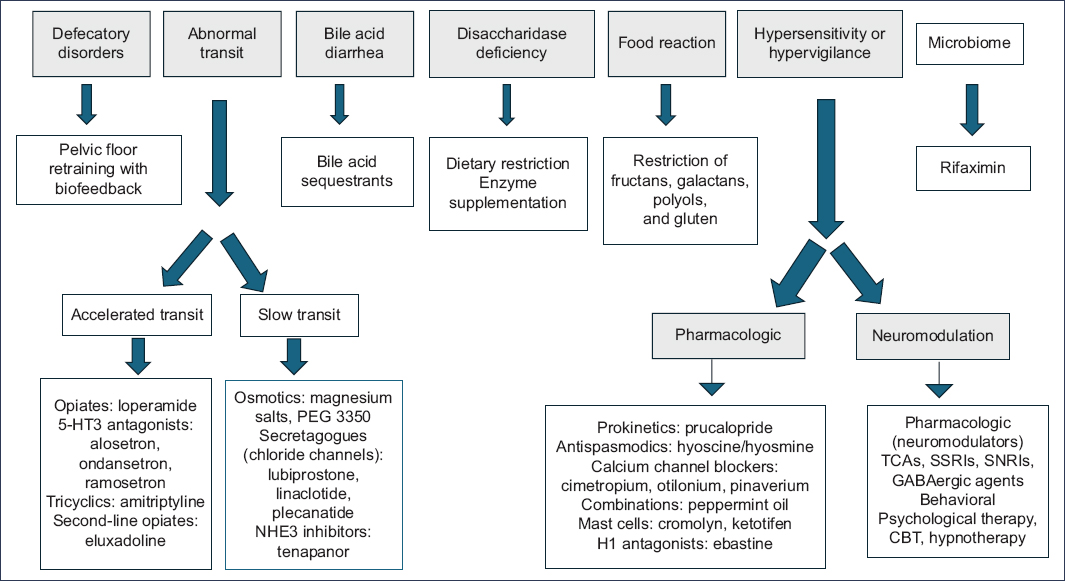
Figure 1. Treatment of irritable bowel syndrome according to endophenotypes. TCA: tricyclic antidepressants; GABA: gamma-aminobutyric acid; SNRI: serotonin and norepinephrine reuptake inhibitors; SSRI: selective serotonin reuptake inhibitors; CBT: cognitive behavioral therapy.
Knowledge regarding the phenotypes, endophenotypes, and subtypes of IBS is of utmost importance for individualizing treatment in each patient and allows us to develop better strategies for follow-up in each case. With respect to initial measures, current clinical practice guidelines on the management of IBS prioritize education, the physician-patient relationship, dietary recommendations, and symptomatic treatments, such as osmotic laxatives for constipation, loperamide for diarrhea, antispasmodics for pain control, and even psychotherapy. Some guidelines prioritize pharmacological treatment and brain–gut behavioral therapy for moderate-to-severe IBS. Therefore, according to the mechanisms and biomarkers of IBS previously described, using a symptom-based algorithmic approach may be of limited utility in optimizing IBS treatment40.
Dietary measures include increased intake of soluble fiber, a low-FODMAP diet (Fermentable Oligosaccharides, Disaccharides, Monosaccharides, and Polyols), and a gluten-free diet. Soluble fiber, particularly psyllium, is more effective than insoluble fiber for patients with IBS-C. Several small clinical trials and systematic reviews and meta-analyses on the low-FODMAP diet exist; however, its efficacy, vs placebo or other diets such as the NICE diet and the British Dietetic Association diet, is similar41. On the other hand, various studies show that a low-FODMAP diet may reduce endotoxin transport across the mucosa in animal models, decreasing abdominal muscle contraction in response to rectal mucosal exposure to stool from IBS patients with elevated endotoxin levels42. Furthermore, IBS patients have large amounts of urinary histamine, suggesting it may be a biomarker for response to a low-FODMAP diet or that they may benefit from H1R antagonists43.
Regarding the gluten-free diet, no benefit has been demonstrated in IBS patients. Two controlled clinical trials, including 111 participants, showed a reduction in the risk of symptom recurrence when gluten-containing diets were reintroduced after a gluten-restricted diet44.
A novel approach to correcting sucrase-isomaltase deficiency, similar to lactase supplementation in hypolactasia, is the administration of sacrosidase, a commercially available enzyme that may reduce symptoms in IBS45.
In clinical practice, pharmacological agents are frequently prescribed for IBS patients for pain management. Antispasmodics are effective, but evidence of their efficacy is limited. Calcium channel blockers and peppermint oil are the most effective agents, but their availability is limited. One study found that ebastine, an H1 receptor blocker, reduced pain in IBS patients46. On the other hand, centrally acting neuromodulators, such as antidepressants, are widely used and recommended in IBS treatment; however, the evidence for their efficacy is weak due to the limited number of clinical trials, as well as biases and overestimation of their effectiveness. PEG 3350, which is a first-line therapy for chronic constipation, has not been formally evaluated in IBS-C. Several studies, however, have evaluated chloride secretagogues (lubiprostone, linaclotide, plecanatide) and the type 3 sodium-hydrogen exchanger inhibitor (tenapanor), which have shown efficacy and have been approved by the FDA for the treatment of IBS-C in patients younger than 65 years without cardiovascular disease47.
Loperamide is the first-line therapy for IBS-D, but no large clinical trials have been conducted in IBS patients. Although eluxadoline acts on opioid receptors and reduces diarrhea it is contraindicated in patients with cholecystectomy. 5-HT3 receptor antagonists are effective in IBS-D48. With respect to non-absorbable antibiotics, rifaximin is more effective for global symptoms and abdominal distension, and it has been approved by the FDA for the treatment of IBS-D49. Regarding fecal microbiota transplantation, systematic reviews and meta-analyses show contrasting results regarding its efficacy in IBS50.
The wide availability of noninvasive clinical tests to determine the mechanisms underlying IBS symptoms is an opportunity to advance individualized treatment, guided by pathophysiology and identified clinical biomarkers.
Irritable bowel syndrome in special populations
Children
Disorders of the brain-gut axis are common in children and adolescents, causing GI symptoms that warrant health care utilization, psychosocial distress, and school absenteeism51. The exact pathophysiology of brain-gut axis disorders is unclear and has a multifactorial origin, involving both internal and external factors. Internal factors include genetic susceptibility, history of infection, intestinal inflammation, microbiome abnormalities, psychological disorders, adverse childhood experiences, and pain response52. In addition, external stressors, cultural factors, and caregiver responses to symptoms may also be important risk factors. For example, a child may develop IBS-C following an enteric infection that causes inflammation and modifies the intestinal mucosa. The psychological interpretation of symptoms, such as abdominal pain, as a threat to well-being—especially in the context of adverse childhood experiences—can exacerbate symptom severity. If a caregiver asks excessive questions, seeks medical care frequently, and causes distress in the child, the child may experience persistent and significant symptoms, as well as poor performance and developmental impairment.
Regarding epidemiology, the global prevalence of IBS in the pediatric population is 8.8%53, being highest in South America (16.8%) and Asia (16.5%), while in Europe it is 10.5%.
With respect to quality of life, children with brain-gut axis disorders score lower than healthy children. These disorders are the second most frequent cause of school absenteeism, underscoring their prevalence and negative impact on quality of life and functioning54.
For the diagnosis of IBS, minimally invasive tests are available, such as serological and stool tests, breath tests, and radiographic studies, as well as more invasive procedures such as endoscopy, manometry, and pH-impedance testing. A stepwise approach should be considered based on the patient’s symptoms and clinical suspicion, in addition to adequately informing patients and their caregivers about the tests requested, since the majority will yield no abnormalities and will not provide a diagnostic conclusion. Therefore, the diagnosis of IBS is based on a detailed medical history and physical examination, and the application of the Rome IV diagnostic criteria. Serology for celiac disease with total IgA levels and IgA anti-tissue transglutaminase antibodies are appropriate if there is evidence of delayed development, failure to thrive, or weight loss. These may also be considered when a child has chronic abdominal pain and changes in bowel habits, particularly chronic diarrhea55.
The biopsychosocial model is a valuable tool for conceptualizing and treating IBS, integrating biological, social, and psychological aspects of the disease. This approach allows for the appropriate identification of psychological or psychiatric comorbidity and its timely management56.
In terms of treatment, lifestyle changes are recommended to avoid polypharmacy and adverse drug effects. Increasing positive activities and reducing negative ones can restore functionality and protect against social, school, and occupational disability. Health professionals can advise patients to engage in activities, foster emotional intelligence, and reduce school absenteeism57. Regarding dietary changes, no specific recommendations are made, as evidence is limited. On the other hand, psychological therapy, such as cognitive-behavioral therapy and hypnotherapy, is useful in many patients and has sufficient evidence of efficacy in the treatment of IBS in the pediatric population58.
As for probiotics, several studies have shown inconsistent results with Lactobacillus rhamnosus GG and Lactobacillus reuteri in IBS, and therefore systematic use in children is ill-advised59. Regarding pharmacological treatment, drugs acting peripherally, centrally, and on the enteric nervous system may be used. In the United States, antispasmodics such as hyoscyamine and dicyclomine are used. These drugs relax intestinal smooth muscle through anticholinergic mechanisms to reduce pain. Although there is insufficient evidence for their use in pediatric IBS, they are prescribed for short periods in cases of abdominal pain. One adverse effect is constipation, which may limit their use when present60. Other treatment options are neuromodulators, which are drugs with effects on the central and peripheral nervous system, with the main function of reducing the intensity of pain and GI symptoms. Although tricyclic antidepressants improve refractory GI symptoms, they prolong the QT interval and are therefore contraindicated in patients with heart disease. Escitalopram is the most studied selective serotonin reuptake inhibitor (SSRI) and is useful for treating depression, anxiety, and obsessive–compulsive disorder. Serotonin-norepinephrine reuptake inhibitors (SNRIs), such as venlafaxine and duloxetine, improve pain and mood disorders and are FDA-approved for the treatment of generalized anxiety disorder. Evidence for their use in pediatrics is limited, but their effects are similar to those of amitriptyline; therefore, they are used in other chronic disorders where pain is the predominant symptom61.
Laxatives and antidiarrheal agents are frequently used in pediatric patients but should be administered with caution because they can exacerbate diarrhea and constipation, respectively. The most widely used laxatives in pediatrics are polyethylene glycol, lactulose, milk of magnesia, bisacodyl, and senna. With respect to antidiarrheals, loperamide and bismuth subsalicylate are commonly used. However, antidiarrheals are contraindicated in cases of enteric infections or inflammatory bowel disease. Among new therapeutic options are linaclotide (a guanylate cyclase-C agonist) and bile acid sequestrants (cholestyramine and colestipol). For the management of IBS-D, new options include bovine serum–derived protein/immunoglobulin isolate and glutamine62.
Older adults
In older adults, the impact of IBS has not been widely studied and its manifestations are not clear. Epidemiological studies suggest that the prevalence of IBS decreases with age, possibly due to changes in pain perception, but it remains a common GI condition in the elderly. Unfortunately, few studies have evaluated the risk factors, diagnosis, and treatment of IBS in older adults. There are reasons to think it behaves differently in older adults and that treatment should be age-based. According to a Danish study, the prevalence of IBS is 6-18%, depending on the definition and clinical criteria used; at the 5-years follow-up, 50-79% of subjects who initially met IBS criteria no longer did so63. In a study conducted in Olmsted County (MN, United States), the prevalence of IBS was found to be 10.9% using the Manning criteria64.
One in 5 patients with IBS has a history of acute gastroenteritis, and according to studies in older adults, the risk is lower vs younger patients. Neal et al.63 studied 544 patients from the Nottingham Health Authority in the UK, recording GI symptoms during 6 months before and after the confirmation of bacterial gastroenteritis. The diagnosis of IBS was established by 2 expert physicians, with a 72% interobserver agreement. One in 4 subjects had persistent intestinal symptoms, and one in 14 developed IBS. Regarding prognosis, IBS in older adults follows a favorable course, as confirmed in a 5-year follow-up study38, in which 50-79% of patients ceased to present symptoms during follow-up.
Although psychological factors may influence the natural history of IBS in younger patients, this has not been demonstrated in older adults65. In a 16-month follow-up study in young patients, the presence of stressful life events was a predictor of symptom severity, and those who improved did not have such antecedents66. A study of 1,119 older adults found that IBS and dyspepsia were more frequent in those with decreased physical and cognitive function, and that IBS was associated with decreased functionality at the 5-year follow-up67.
Although differential diagnosis of colonic symptoms in older adults is similar to that in younger patients, organic diseases are more frequent in this age group. Colonoscopy is important to rule out colorectal cancer and other conditions68.
Treatment of IBS in older adults remains empirical and symptomatic, with emphasis on dietary changes and physical activity as first-line therapy. Soluble fiber intake should be increased gradually to minimize intolerance. In addition, dehydration is common in older adults, and patients should be advised to maintain adequate hydration69.
IBS in older adults is underdiagnosed and under-researched, despite its major economic and quality-of-life impact.
Pregnant women
IBS has a high prevalence in pregnant women due to its frequency in women of reproductive age. Pregnancy can exacerbate IBS symptoms, with an 11-38% increase in constipation—especially in the third trimester—but 34% report increased bowel movement frequency70.
Pregnancy exacerbates obsessive-compulsive disorder and increases hypochondriacal behavior and illness phobia. Stress increases in the third trimester, which may predispose to exacerbation of IBS symptoms71.
Regarding intestinal function during pregnancy, studies in humans and animal models show changes in GI motor function, with prolonged orocecal transit in humans and delayed transit in mice72. Pregnancy hormones, particularly estrogens and progesterone, impact GI function, potentially worsening IBS symptoms. Although these hormones may slow intestinal transit and gastric emptying, they have an analgesic effect too that may decrease IBS pain.
For treatment during pregnancy, dietary changes are recommended, particularly fiber supplementation. Psyllium may increase stool consistency, decrease intestinal transit time, and improve constipation. However, the effectiveness of fiber supplementation varies depending on the type of fiber and the specific IBS symptoms73. Treatment of IBS during pregnancy prioritizes dietary changes and psychological interventions over pharmacological options, as most available drugs fall within FDA categories B, C, or D. Osmotic laxatives, including lactulose and polyethylene glycol, are effective for constipation in pregnancy; lactulose improves stool frequency and consistency after 2 weeks. Other laxatives, such as docusate, bisacodyl, senna, and phenolphthalein, are also safe; dantron may be teratogenic. Hypertonic saline laxatives should be used with caution due to the risk of electrolyte disturbances74. For IBS-D, peripherally acting opioids are the initial treatment in non-pregnant patients. Loperamide is generally safe during pregnancy, while diphenoxylate with atropine should be avoided due to teratogenic effects75. Other drug classes, such as antispasmodics, anticholinergics, and calcium channel blockers, are frequently prescribed for IBS, particularly in cases of abdominal pain. However, their use during pregnancy is limited due to potential risks, especially with anticholinergics76. Regarding neuromodulators, tricyclic antidepressants are effective for IBS symptoms in non-pregnant women; amitriptyline, trimipramine, and desipramine show benefits for multiple symptoms, and safety studies of antidepressants in pregnancy show no increase in fetal malformations or long-term adverse outcomes. However, potential risks should be considered, and these agents should be prescribed only in cases of severe IBS symptoms during pregnancy77. Other agents, such as simethicone, activated charcoal, and pancreatic enzymes, may reduce abdominal bloating in IBS, but their use during pregnancy has not been studied78. On the other hand, psychological therapies—including cognitive-behavioral therapy, hypnosis, and muscle relaxation training—have shown promising results in the treatment of symptoms and in improving quality of life. These therapies are particularly beneficial in pregnant women with severe symptoms. Complementary and alternative medicine for IBS, such as traditional Chinese herbal medicine, shows variable results in non-pregnant women, but its safety and efficacy during pregnancy are unknown, and therefore its use is not recommended79.
Conclusions
Proper identification of IBS subtypes and phenotypes is of utmost importance, as it enables individualized approaches and treatment that positively impact symptom relief and quality of life. Treatment options include dietary modifications and pharmacological therapy, such as laxatives, antidiarrheals, antispasmodics, and neuromodulators. Non-pharmacological options, such as psychological therapies, are effective in certain patients, particularly those with a history of adverse events early in life. It is also essential to approach IBS according to age group, since treatment and response may vary depending on the characteristics of each special population.
Funding
The author declared having received no funding for this study.
Conflicts of interest
The author declared no conflicts of interest whatsoever.
Ethical considerations
Protection of humans and animals. The author declares that no experiments on humans or animals were performed for this research.
Confidentiality, informed consent, and ethical approval. This study does not involve patient personal data and does not require ethical approval. The SAGER guidelines do not apply.
Declaration on the use of artificial intelligence. The author declares that no generative artificial intelligence was used in the preparation of this manuscript.
